Archived News Articles
New and Updated Methods at NQAC Dublin
Deoxynivalenol-3-Glucoside (DON-3-G)
NQAC Dublin recently updated our mycotoxin screen to include Deoxynivalenol-3-Glucoside. DON-3-G is one of the primary metabolites of deoxynivalenol (DON), and is of interest when evaluating the total deoxynivalenol content of a sample. The limit of quantification is 50 µg/kg with a routine turnaround time of 7 days. A rush turnaround time is also available at 5 days.
Tropane Alkaloids
Tropane Alkaloids (TA) are secondary metabolites naturally occurring in several plant families, including Solanaceae and Brassicaceae. Although over 200 tropane alkaloids are known, (-)-scopolamine and (-)-hyoscyamine are the main alkaloids produced. (-)-hyoscyamine undergoes racemization over time and the racemic mixture of is called atropine. This method provides quantitative analysis for tropane alkaloids, atropine and scopolamine.
This method has a limit of quantitation of 1 µg/kg for oil and 0.5 µg/kg for all other matrices. Routine turnaround time for this method is 7 days and a rush turnaround time is 5 days.
Human Milk Oligosaccharides (HMO)
Human Milk Oligosaccharides (HMOs) are the third most abundant solid component of breast milk, after fat and lactose. They play a key role in the healthy development of babies, preventing infections, and promoting the growth of healthy bacteria.
Our HMO in Infant Formula analysis has been expanded to include difucosyllactose, lacto-n-tetraose, lacto-n-neotetraose, 3’-sialyllactose, and 6’-sialyllactose. The turnaround time for this method is set at a routine turnaround of 14 days and a rush turnaround of 10 days. The limit of quantitation varies by component.
Vicine and Convicine Testing in Fava Bean Derived Ingredients
Over the past few years, the popularity of plant-based foods has skyrocketed leading to an explosion of new product development, innovation, and brand launches in this category. Recent data commissioned by The Good Food Institute shows that retail sales of plant-based foods intended to replace animal-based foods have grown over 30% in a two-year period, reaching nearly $4.5 billion in sales as of 2019.1
With this growing interest in vegan and vegetarian foods, manufacturers are investigating plant-based proteins to use in product development. Fava beans (Vicia faba) have been increasingly used in the development of these alternative protein sources and have been suggested as an alternative to soybeans based on its potentially smaller environmental impact.2 Fava beans are adapted to a wide range of global agro-ecological zones which reduces greenhouse gas emissions that result from transport over long distances. They are also able to symbiotically fix nitrogen which reduces the reliance on nitrogen inputs into the soil and allows for improved soil fertility.3 From the product development aspect, fava beans provide color, taste and texture that is competitive with soy and other plant-based protein alternatives. This makes it an attractive option due to its bright color and neutral taste.2
Despite the positive aspects of using fava beans as an alternative protein, the potential presence of vicine and convicine has traditionally restricted its use. Vicine and convicine are alkaloid β-glycosides found in fava beans (Vicia faba) that are hydrolyzed to their aglycones (divicine and isouramil) in the colon. These two aglycones are toxic to individuals who suffer from hereditary loss of the enzyme glucose-6-phosphate dehydrogenase. This toxicity develops into a potentially fatal form of anemia known as favism.
This risk can be mitigated through adequate processing and treatment, but it is also essential that raw material and finished products are monitored for the presence of these compounds. NQAC Dublin recently implemented a method that allows for the quantitative determination of vicine and convicine in fava beans and food products containing fava bean derived ingredients. We utilize UPLC-UV technology with a BEH-HILIC column to analyze vicine and convicine with a quantifiable limit of 20 mg/kg for raw materials. Finished products can be tested with a quantifiable limit of 2 mg/kg, however at this time these products are not fully validated with the method.
If you have questions or would like to discuss vicine and convicine testing and your product needs, please reach out to nqacdublincustomerservice@us.nestle.com and we’d be happy to answer any questions you have.
- https://www.forbes.com/sites/douglasyu/2020/01/19/plant-based-foods-are-hot-now-they-just-got-hotter/?sh=11816f13214c
- https://www.foodingredientsfirst.com/news/are-fava-beans-the-new-soy-danish-scientists-highlight-crop-that-bursts-with-protein-296799.html
- http://centaur.reading.ac.uk/88530/8/1-s2.0-S0924224419301992-main.pdf
June 7th is World Food Safety Day!
In 2018, the United Nations General Assembly announced that every June 7th would be World Food Safety Day (WFSD). The goal of WFSD is to increase awareness of issues impacting food security, human health, and economic prosperity, and to encourage action in preventing, detecting, and managing foodborne risks worldwide. The theme for 2021 is “Safe food today for a healthy tomorrow”; emphasizing the importance of the production and consumption of safe food and the direct effect it has on the health of people, the environment, animals, plants and the economy.1
At NQAC Dublin, food safety is at the heart of what we do. From routine microbiological and contaminant monitoring of products to assisting with setting up environmental monitoring programs, NQAC Dublin is here to assist you every step of the way.
Please reach out to our customer service team at nqacdublincustomerservice@us.nestle.com to discuss how we can help you reach your food safety goals in 2021.
- https://www.who.int/news-room/campaigns/world-food-safety-day/2021
Advantages of using a molecular method to Serotype Salmonella
By: Carol Sivey – Microbiologist
What is manual serotyping?
Salmonellae are rod-shaped bacteria that have many hair-like structures, known as flagella, extending from their cell wall to give them motility. The flagella and cell walls of the organism contain proteins called antigens that are used to distinguish between different Salmonella serovars. Manual serotyping involves growing the cells and then adding antisera onto either a glass slide or into a test tube along with the cultured cells. If an antisera “matches” an antigen present in the sample, they fit together like a lock and key and fall out of solution since this combination is heavier than the surrounding particles. This is known as agglutination and identifies which antigens are present on the Salmonella being typed.
Does manual serotyping take a long time?
If there are no complications, serotyping can be completed rather quickly! Once the Salmonella is isolated, the antigens on the cell wall (known as “O” antigens) are characterized by dropping reagents on a glass slide. This requires some skill from the analyst to know how much culture to mix with the antisera but can usually be completed in minutes and we don’t often see complications.
Characterizing antigens on the flagellae (known as “H” antigens) can be more challenging. Most Salmonellae have two phases of their “H” antigens. To determine the first phase, the culture is re-grown for several hours in a broth. This broth is then diluted and added to tubes containing different antisera. The “H” antisera are determined by which of these test culture tubes show agglutination. Since most Salmonella species have two phases of their “H” antigen this, process must be repeated to determine the second phase. To do this, the isolate is regrown in media containing 1st phase antigens so that the culture is forced to revert to the phase 2 antigen to grow. Then the same process is repeated to determine the phase 2 antigens.
Once these 3 antigens are obtained, a table is consulted to match the “O”, 1st “H”, and 2nd “H” to a serotype name, such as “Salmonella typhimurium”.
In practice, however, serotyping is rarely so cooperative. The isolate may have weakened flagellae, perhaps due to food processing techniques. In these cases, the “H” serology isn’t possible until we repair the flagellae by transferring the culture through an enriched medium a few times. This adds days to our result timeline.
Additionally, sometimes the culture has been infected by a virus known as a bacterial phage which makes serological falls impossible. In these cases, we return to the original isolation plate to hopefully choose another colony to work with that hasn’t been infected. This re-start also adds additional days to the process.
Furthermore, we occasionally encounter isolates that prefer one “H” phase over another. In these cases, when the lab tries to reverse phases, the organism continues to revert to the phase it prefers. To try to counteract this, the lab may reduce incubation times in the broth regrowth step to catch it in the second phase before it returns to its favorite phase. This again adds additional turnaround time to the serotyping process.
What is molecular serotyping and how does it improve TAT?
NQAC Dublin recently implemented Salmonella Serotyping by PCR. using the Check&Trace™ Salmonella assay by Check-Points. This method uses molecular techniques to serotype Salmonella based on DNA composition. Target DNA sequences are first captured and amplified. The amplified sequences are placed on a DNA microarray; a microchip containing probes that bind specifically with these sequences. After staining, a pattern of dots develops on the microchip. The dots are then analyzed by computer software for comparison with patterns of reference.
While, molecular serotyping isn’t the best choice for all isolates, for common serotypes, the time savings is great. Once an isolate is obtained, this assay can provide a serotype in 8 hours.
Perhaps the real time-savings occurs for the problematic strains mentioned earlier. If the isolate still possesses the genes for flagellae, weakened flagellae and phase “stubborn” strains should not be a problem for a molecular assay. Phage infection may not be a problem either if the molecular regions that the assay targets are not compromised by the virus.
If you have questions or would like to discuss serotyping methods and your product needs, please reach out to nqacdublincustomerservice@us.nestle.com and we’d be happy to answer any questions you have.
Understanding Added Sugars
In 2016, the Food and Drug Administration (FDA) amended the labeling regulations for foods and dietary supplements to assist consumers in making healthy dietary choices. Part of this revision was the inclusion of a declaration for “added sugars” on the nutritional label.1
Added sugars are defined by the FDA as sugars that are added during food processing and include:
- Sugars (free, mono-, and disaccharides)
- Syrups
- Naturally occurring sugars that are isolated from whole foods and concentrated (e.g. fruit juice concentrates)
- Caloric sweeteners1
Excluded from this designation are:
- Fruit and vegetable juices that are un-concentrated
- Fruit and vegetable juices concentrated from 100 % fruit juice sold directly to consumers
- Dried fruits and vegetables
- Fruit component of fruit spreads2
How do I determine the “added sugars” value for my product?
Unfortunately, there is no way to analytically measure “added sugars”, as they are not discernable from total sugars in a product through laboratory testing. To determine the “added sugars” value for label declaration, it is necessary to conduct a careful evaluation of product ingredients and understand how the manufacturing process may affect the final sugar content of the product. This value can be compared to the total sugars content from laboratory testing to complete the Nutrition Facts Label.
Why is the total sugars value on my analytical report different from what was expected?
Certain manufacturing processes can have an impact on the total sugars value for a product. For example, if a manufacturing process includes non-enzymatic browning (NEB), such as caramelization or Maillard Browning, or fermentation, the total sugar content of the final product could be reduced. Conversely, if manufacturing includes any hydrolysis processing, the breakdown of complex carbohydrates into mono and disaccharides can potentially contribute to an increase in the total sugars value of a product.
Due to this, it is possible that the total sugars value reported out through laboratory testing could be lower than the added sugars value you intend to declare on your label. Unfortunately, FDA regulation does not allow for product labeling with an added sugars value that is larger than the total sugars value. In this case, FDA guidance advises that the amount of added sugars declared should equal the amount of total sugars reported through analytical analysis.3
If you have any questions or would like additional assistance with nutritional labeling, please reach out to us at nqacdublincustomerservice@us.nestle.com we’d be happy to answer any questions you have.
References
1) Nutrition labeling of food, 21 C.F.R. § 101.9 (2019, April 1). Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.9
2)United States Department of Agriculture. (n.d.). Dietary Guidelines for Americans 2015-2020 Eighth Edition. Retrieved from https://www.dietaryguidelines.gov/
3)United States Food and Drug Administration. (2019, December). Guidance for Industry: Nutrition and Supplement Facts Labels Questions and Answers Related to the Compliance Date, Added Sugars, and Declaration of Quantitative Amounts of Vitamins and Minerals. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-nutrition-and-supplement-facts-labels-questions-and-answers-related-compliance
The “Dirty Dozen” 2021: How can you ensure your product is safe?
The Environmental Working Group (EWG) recently released an updated “Dirty Dozen” list of conventional fruits and vegetables most heavily contaminated with pesticides. This list is based on EWG’s analysis of the latest test data from the Department of Agriculture, which shows that roughly 70% of conventionally grown fresh produce sold in the United States contains some level of pesticide residues (1).
“Dirty Dozen” for 2021:
- Strawberries
- Spinach
- Kale, Collard and Mustard Greens
- Nectarines
- Apples
- Grapes
- Cherries
- Peaches
- Pears
- Bell and Hot Peppers
- Celery
- Tomatoes
This year, EWG has placed additional focus on fungicides in citrus products that are not directly captured on the “Dirty Dozen” list. Over 95% of tangerines tested by the USDA and 90% of the citrus fruits tested by EWG contained imazalil residues. This fungicide is classified by the EPA as a likely human carcinogen and can change hormone levels (1).
If you are using conventionally grown fruits and vegetables in your products, you may be wondering what you can do to mitigate this risk and ensure your products are meeting regulatory guidelines. One-way to help ensure a safe product is by developing a robust contaminant-testing plan, which includes a screening for the major pesticides and fungicides used in crop production. NQAC Dublin offers a wide range of pesticides analyses that can help your facility understand any potential pesticide residues present in your raw materials.
NQA-54.0003 – Pesticide Residue Analysis by Electrospray LC-MS/MS – Chem
NQA-54.0005 – Pesticide Residue Analysis by GC-MS/MS – Chem
LI-00.039 – Glyphosate, AMPA and Glufosinate (FMOC) – Chem
If you have any questions or would like to discuss our pesticides testing options, please reach out to us at nqacdublincustomerservice@us.nestle.com we’d be happy to answer any questions you have.
References:
New Food Magazine: Understanding the capabilities of your lab method
Fitness for purpose of an analytical method is essential in ensuring product compliance to regulatory and production limits. The validation of an analytical method can give important indications into the result variation of a method but other variables, such as raw materials and production processes, can also affect the fitness for purpose of a method. For this reason, the key performance characteristics for analytical methods, as well as process capability and its link to method variability, should be taken into consideration when reviewing the fitness for purpose of an analytical method.
In the latest issue of New Food Magazine, Fabien Robert, Head of Zone America Nestle Quality Assurance Centers, and Erik Konings, Program Manager at Nestle Research, review these key concepts as well as discuss a holistic approach that can be used to ensure the fitness for purpose of an analytical method.
To learn more about this critical concept for food manufacturers, the full article can be found starting on pg. 50 of Issue 1 2021 of New Food Magazine, or at newfoodmagazine.com below!
https://www.newfoodmagazine.com/article/138168/nestle-talks-analytical-methods/
Excellent 2020 Proficiency Test Performance
A commitment to quality is embedded in everything we do at NQAC Dublin. Demonstrated by our culture, daily work of our staff, and the quality programs we have in place, including our participation in a proficiency program.
Participation in a proficiency program not only meets GLP and regulatory requirements, but shows competency of staff and accuracy of analytical methods.
Our proficiency test performance has always been highly recognized. Each year our results have exceeded the industry standard and in 2020, our performance was exemplarily. We achieved 0% unsatisfactory results out of 267 tests performed under the proficiency program.
We are proud of our team and their outstanding performance. At NQAC Dublin, we work everyday to ensure our customers feel confident in the results they receive from us.
If you would like more information on our proficiency test performance or have questions, contact us today and we’d be happy to discuss in more detail: nqacdublincustomerservice@us.nestle.com.
Expert Support for your 2021 Food Safety Programs
A strong food safety program is critical to the success of your business and for the safety of your consumers. This program should be a “living document” that is revised and updated as your business grows and adapts to changes in the food industry. Food safety experts have seen the most success when companies follow a three-step process when creating or revising an existing plan:
- Assemble a team to focus on food safety, including top management
- Build a strong HACCP plan
- Select a certification standard to guide your implementation process1
If this process seems overwhelming, or you would like to ensure that your food safety program is complaint and robust, NQAC Dublin offers a wide range of support ranging from laboratory testing to risk assessments and audit support.

We provide accurate results, quick turnaround times and competitive pricing with industry-leading service to support your food safety and quality assurance needs.
If you have more questions or would like to discuss services and support options, please reach out to us at nqacdublincustomerservice@us.nestle.com we’d be happy to answer any questions you have.
REFERENCES:
(1) https://foodsafetytech.com/column/where-to-start-on-your-companys-food-safety-program/
Is it really necessary to test at a higher dilution for pathogen testing?
By: Carol Sivey – Microbiologist
Why do laboratories sometimes require high dilutions when screening for pathogens like Salmonella and Listeria?
Spices and flavorings often contain essential oils that inhibit bacteria, including pathogens, from growing to detectable levels. Furthermore, in the case of spices and herbs, the concentration of inhibitory substances can vary for the same spice depending on the region of the world and the season of the year in which it was grown. This can create a challenge for pathogen detection in these materials. If bacterial growth is not inhibited, target pathogens can usually grow to 106 /mL or higher in an overnight enrichment medium, with most next-day screening platforms requiring a concentration of at least 103 /mL or higher for accurate detection. If these levels are not achieved, a false negative result is possible.
This inhibitory activity of spices, herbs, and flavorings can be overcome by diluting the sample beyond the standard testing dilution, which would allow for a more favorable environment for bacterial growth.
Often, for their intended applications, spices and flavorings are used in foods at relatively low levels and higher testing dilution mimics the level that these materials are present in consumer goods. At these levels, their inhibitory agents can be diluted beyond toxic levels and pathogens can grow into a threat to food safety. The laboratory must therefore conduct the screening at a concentration that has been proven to allow for the growth of Salmonella and Listeria.
NQAC Dublin has an on-going pathogen verification program. Trial dilutions of a matrix are spiked with low levels of Salmonella or Listeria, tested per respective protocols, and the lowest dilution that achieves recovery becomes the test dilution for that matrix. We currently have over 500 different matrices in our verification database.
Is there any research into developing enrichment medias to overcome inhibitory substances?
Yes, and our technical team is actively participating in these studies! We understand that high dilutions of up to 1:1000 or higher, significantly raise the cost of food safety. Our team recently participated in an ISO study to evaluate neutralizers to enhance recovery of Salmonella from spices. Five neutralizing agents were tested for efficacy in oregano, cinnamon, and cloves. The spices were spiked with low levels of stressed Salmonella and recovery was detected culturally based on ISO 6579-1:2017. One item, activated charcoal, proved effective in reducing oregano dilutions from 1:100 to 1:20, cinnamon dilutions from 1:200 to 1:50, and cloves from 1:500 to 1:100. While not yet validated and not in routine use at our laboratory, we hope to further these studies with a media vendor or another laboratory to work toward full validation. The study was summarized in a poster presented at the 2020 Virtual IAFP Annual Meeting in late October: Neutralization of Inhibitory Substances in Oregano, Cinnamon, and Cloves for the Recovery of Salmonella. C. Sivey, D. Tomas, A. Finnarn, and K. Sow.
So yes, it really is necessary sometimes for pathogen testing to require higher dilutions, especially when it comes to inhibitory matrices like spices, herbs and flavorings. The higher dilutions are proven to achieve the most accurate results and lessen the potential for a false negative result being reported for your product.
Ensuring accurate results, especially when it comes to pathogen testing, is the highest priority for NQAC Dublin. It’s an important responsibility to ensure that the food, beverages, and ingredients tested in our facility are held to the highest standards. It’s part of our responsibility to help protect both consumers and food industry businesses. To that end, we hold all the products we test to the utmost safety and quality standards.
If you have more questions or would like to discuss our verification programs and your product needs. Contact us and we’d be happy to answer any questions you have.
Selecting the appropriate method is critical for ensuring accurate results for your premix sample. Here are some steps to guide you through appropriate test selection:
By: Jessica Hanson – Chemist
When selecting testing for a sample, it is important to identify a few aspects about the sample matrix in order to help ensure your results are accurate and timely.
- Is the sample a premix or finished product?
A premix is a blend of micronutrients (vitamins and minerals) used to fortify and enrich food products; thus, its concentration of micronutrients is very high. A finished product typically has a much lower concentration of micronutrients.
Determining which category your sample falls into is an essential first step in selecting the appropriate method for testing and will help ensure results are issued as quickly as possible. If you are unsure, supplying the laboratory with accurate estimated levels for the vitamin in question will help the laboratory in updating the testing, if necessary, prior to performing any analytical work.
As an example, suppose the lab receives a sample requesting Vitamin B12 testing. The method selected for testing is intended for products with lower concentrations of vitamin B12 and no estimated levels are supplied to the lab prior to testing. The laboratory preps the sample for the finished product B12 method and performs the testing, however when the results are generated it is found that they are outside of the calibration curve1 due to high concentration of B12 in the sample. The sample will need to be re-prepped and tested using the premix B12 method, which delays the issuance of final results.
- What form of micronutrient is present in the sample?
Providing the form of the micronutrient in the sample can give the laboratory valuable information when determining if the method selected for testing is most appropriate for the sample type. In certain instances, the form of vitamin present in the sample may require testing under a different methodology.
Going back to the Vitamin B12 example, the sample being tested contained methylcobalamin but this information was not provided prior to testing. The laboratory did the re-prep and retest using the premix method for B12 based on the over the curve results obtained during the initial round of testing. However, this time the results are coming back as not detectable. In this case, the sample should have been tested using the finished product method that can account for methylcobalamin with additional dilutions2 added during prep due to the increased concentration of B12 in the sample. The sample will need to be re-prepped a third time and retested, resulting in additional delay in result reporting.
As you can see, identifying if the product is a premix, providing estimated levels, and specifying the form of micronutrient can reduce the time it takes to get sample results as the correct method can be selected during the initial analysis and any necessary dilutions can be performed.
NQAC Dublin offers a wide variety of vitamins methods covering many different product types and ingredients. If you desire help in selecting which test(s) are best for your needs, our Customer Service team (nqacdublincustomerservice@us.nestle.com) is available to provide information and assistance.
What could be worse than opening the refrigerator only to discover food covered in furry white (or blue, or green) mold?
By: Ernest Capraro – Chemist
Although the visual is repulsive, far worse is unknowingly eating food containing mold and the toxins that certain varieties produce.
Mycotoxins (the prefix “myco” comes from Greek, and refers to fungi/mold) are pervasive and represent a constant threat to the world’s food supply. The mycotoxins they produce remain even if the fungus is killed. These toxins vary in effect. With its readily apparent symptoms, Deoxynivalenol (DON) is commonly known as vomitoxin, presenting an acutely severe health risk. On the other hand, Aflatoxin B1 is the world’s most potent naturally occurring carcinogen, posing a significant chronic risk. The USDA has made available a detailed mycotoxin guide which includes the health effects of a wide number of mycotoxins.
Mycotoxins are harmful to both humans and livestock. Monitoring is fundamental to protect the integrity of the food supply network, and the health of consumers. Nations around the world maintain regulations limiting the mycotoxin content in various foods. Limits vary across borders, but one constant is that the strictest (lowest) limits are applied to infant food and formula. Because food production is a global industry, keeping abreast of regulations is key. A user-friendly resource can be found at mycotoxins.info, providing regulations by region and nation.
A mycotoxin monitoring program should be driven by the need to comply with regulations in target markets, and a thorough risk assessment. While the design of such a program is beyond the scope of this article, recognizing some of the biggest threats is not difficult. Consider that fungi will primarily grow in one of two places – in the field, or in storage. “Field mycotoxins” are those that are produced by fungi that grow on live crops. In the case of grains or nuts, this means that the fungus will already be present at the time the seed starts to form, and ultimately becomes trapped inside. “Storage mycotoxins” grow under storage conditions that favor fungal growth. Uncontrolled high humidity or other dampness, especially with minimal air circulation, increase this risk.
Field mycotoxins are most commonly observed on grains. In the Americas, there is a tendency to see DON, Fumonisins B1 and B2 (FB1 and FB2), and Zearalenone (ZEN / ZON) in grains. Corn, in particular, is frequently contaminated by higher levels of these toxins. Wheat is another commonly contaminated grain, while rice typically exhibits lower levels. In nuts, it is most common to observe the Aflatoxins – B1, B2, G1 & G2. The largest Aflatoxin contaminations tend to occur in pecans. Since mycotoxins are not easily destroyed, any foods prepared from these grains or nuts is also at risk. For instance, corn oil could easily contain ZEN.
Storage mycotoxins threaten products that may be stored for a period of time before processing or making it to market. Aflatoxins B1, B2, G1 & G2 and Ochratoxin A (OTA) are the primary storage mycotoxins. Cocoa powder or coffee beans are examples of materials susceptible to fungal growth during storage. Grains, too, may suffer fungal attacks while stored.
One significant mycotoxin isn’t directly produced by a fungus. When a cow eats grass, hay, or other feed that is contaminated with Aflatoxin B1, the cow is able to metabolize some into a new form that is called Aflatoxin M1. The “M” refers to milk, in which the mycotoxin can be found. As such, dairy products are typically monitored for Aflatoxin M1. Most importantly, infant formula needs this testing, since babies are among the most vulnerable to mycotoxins.
NQAC Dublin has testing options for these mycotoxins and more. If you have specific mycotoxin testing requirements, or desire help in selecting which test(s) are best for your needs, our Customer Service team (nqacdublincustomerservice@us.nestle.com) is available to provide information and assistance.
Protect your Customers and Business with Environmental Monitoring
By: Andrea Chmelar – Environmental Monitoring Specialist, Microbiology Supervisor
Pathogens are everywhere and often show up when you least expect it. They have the potential to cause significant damage to your brand, customer trust and, most importantly, consumer health. A well-designed Environmental Monitoring (EM) Program can help mitigate this risk and is an essential component to a robust Pathogen Monitoring Program (PMP). Developing strong environmental monitoring is a journey and takes a lot of work but, when done correctly, it can positively impact your bottom line.
EM provides insight into the microbial population of your factory and verifies that your food safety management systems are working as intended. It should provide you with early pathogen detection and is required by the Food Safety Modernization ACT (FSMA) if you have product that is exposed after a kill step and before packaging. To be clear, EM does not prevent pathogens from entering your product – this is what your hygienic controls and pre-requisite programs are for, but EM will tell you how well you are executing these programs.
There are several components to a healthy EM program:
- site selection and frequency – based on risk and proximity to production line
- tool selection – based on the product manufactured in the factory, sanitizer used in cleaning procedures and product specifications
- data management – accurate records of site results to allow to trending and analysis
- solid corrective action processes – provides guidance for action in the event of a positive pathogen result or out of specification hygiene indicators
- strong training and coaching – your program is only as strong as the training/coaching program supporting it
Remember, having an excellent Environmental Monitoring program takes practice and vigilance. Your program is a living document that may need to adapt to the season, a specific product type, or different areas of your facility.
If you would like learn more about the components of a strong EM program or if you are looking to revamp your current program, contact us today and our quality professionals will discuss your needs and set you on the right path: nqacdublininfo@us.nestle.com
New Methods Available at NQAC Dublin
On 8/10/2020, NQAC Dublin is excited to announce that we will be launching five new additions to our analysis portfolio. Please find details about each new method below and please reach out to us at nqacdublincustomerservice@us.nestle.com if you have any questions.
Quarternary Ammonium Compounds (QAC)
Quarternary Ammonium Compounds is verified and will be available as LI-00.044 Quarternary Ammonium Compounds by LC-MS/MS. This method analyzes for seven quarternary ammonium compounds including DDAC, BAC-8, BAC-10, BAC-12, BAC-14, BAC-16 and BAC-18) in foods.
Details:
- Validated matrices include:
- Powdered Infant Formula (milk, powder) and fresh milk
- Fresh fruit and vegetables (high water content vegetables)
- Validated matrices have a reporting range of 0.010-1.5 mg/kg
Volatile Organic Compounds
Volatile Organic Compounds (VOC) in drinking water is verified at our location and will be available as EPA 524.2 by GC-MS. The method is applicable to finished drinking water, raw source, or drinking water in any treatment stage.
Details:
- A full list of compounds covered by this method is available by request
Osteopontin in Infant Formula
Osteopontin (OPN) is a highly glycosylated, phosphorylated, acidic whey protein that could potentially play a role in infant immunity and development. It is present in human milk at approximately 138 mg/L; a level around eight-times that of bovine milk (18 mg/L). The protein has been implicated in a wide number of biological processes including cell survival, bone remodeling and immune modulatory functions.
Details:
- Osteopontin (OPN) in infant formula and growing up milk by UPLC-MSMS is available under NQA-55.0002
Lutein added to Carotenoids Method
Lutein is in the family of carotenoids found in common foods including spinach, peas, and broccoli. It is a structural component of the eye, potent antioxidant, and will be available for testing under the expanded carotenoids method LI-00.683-2 by HPLC-UV.
Details:
- LI-00.683-2 has been validated for testing on Infant Formula and Adult Nutritionals
- Results will be issued with a range of 20.0-1000 µg/100 on these matrices
Food Nutritional Fortification Substance Lactoferrin
As our final new method announcement, we will now offer a method for the analysis of Lactoferrin listed as a Food Nutritional Fortification Substance, GB 1903.17-2016.
This standard applies to food nutritional fortification substance Lactoferrin prepared using milk and milk products as raw materials through separation, sterilization, extraction, refinement and drying.
Have you ever experienced a foreign body incident in your factory? Or have your customers ever reported finding one in your product?
By: Daniel Smieszek – Technical Services
When purchasing food products, consumers expect products that are high quality, safe and free from any unexpected foreign bodies. A useful working definition of a foreign body is “an object which can be seen by the unaided eye or felt in the mouth, and which the consumer perceives as being alien to the food” [1]. This may include common materials such as metal, plastic, glass, stones and wood. These unexpected materials are typically inadvertently introduced during processing, manufacturing, packaging or shipping. Consumers may also notice other foreign bodies that are part of the food ingredients but undesirable, such as plant leaves/stems, seeds and even scorched product.
Food manufactures must put controls in place to prevent foreign bodies from entering the product but even with safeguards in place, consumers still find them on a frequent basis. A foreign body incident can have huge impact on consumers and can even lead to product recalls. In 2019, the FDA estimated that 6% of recalls were due to foreign bodies [2]. In addition, Food Safety Modernization Act (FSMA) requires that physical hazards are identified and preventive controls are put in place.
 It is important to identify the foreign body quickly and accurately to attain root cause and prevent recurrence. Many foreign bodies can visibly look similar to the unaided eye so it is critical to have the proper technology to support identification.
It is important to identify the foreign body quickly and accurately to attain root cause and prevent recurrence. Many foreign bodies can visibly look similar to the unaided eye so it is critical to have the proper technology to support identification.
We use Fourier Transform Infra-Red Spectroscopy (FTIR) and X-Ray Fluorescence Spectroscopy (XRF) technologies to help identify glass, plastic, metal and organic materials. These technologies generate a customized report that includes microscopic view of the foreign material and techniques are used to help identify or compare your foreign bodies.
Here are some additional details on the two technologies we use:
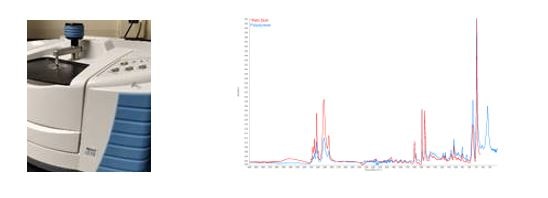
NQA-00.8321 Material Characterization by FTIR
The use of IR radiation to determine chemical bonds & functional groups creating a molecular fingerprint. This fingerprint is compared to known references for identification.
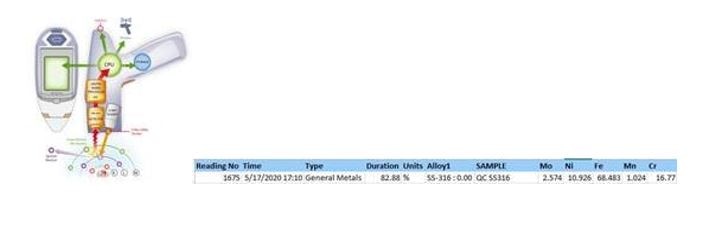
NQA-00.8325 Material Characterization by XRF
The use of X-rays to identify elemental composition for metal & glass. Elemental percentage is used to determine potential metal alloys for the foreign body.
Do you need to know if that white plastic is coming from your factory ? Would you like a custom library of potential sources of foreign material in your facility?
We offer identification of foreign materials as well as mapping of production lines and can develop a custom material characterization library to assist with your management of foreign bodies. By preparing your facility for a foreign body incident, if one occurs, you will have the ability to react quickly to identify the source and prevent any further disruptions and potential losses.
If you need assistance with foreign body investigations or characterizing your consumer complaint samples, please contact our Customer Service team at nqacdublincustomerservice@us.nestle.com for more information.
References:
FDA: New Era of Smarter Food Safety Blueprint
The FDA recently launched their New Era of Smarter Food Safety Blueprint and we are excited to see where the future of food safety leads us. Their approach outlined in the blueprint shares the FDA’s vision over the next decade of their food safety goals.
A modern approach to food safety is outlined in the blueprint with goals of enhanced traceability and predictive analytics. With more transparency, we will have the ability to quickly respond to foodborne illness outbreaks and reduce food contamination. Developing stronger food safety cultures is also one of the core elements of the blueprint.
As our country has navigated through the COVID-19 pandemic, experts have learned many lessons throughout and will use their newfound knowledge to create a safer food system for the future. It will be more important than ever for transparency and partnerships between public health, industry and government authorities as we move into the future of food safety. FSMA has provided the groundwork for the FDA blueprint and moving into the next decade, we’ll see new foods, products, technologies along with established science and risk-based protections guide changes for a safer food system.
The below are the four Core Elements outlined in the blueprint:
- Core Element 1: Tech-Enabled Traceability
- Core Element 2: Smarter Tools and Approaches for Prevention and Outbreak Response
- Core Element 3: New Business Models and Retail Modernization
- Core Element 4: Food Safety Culture
For the full blueprint and all the details, visit: https://www.fda.gov/food/new-era-smarter-food-safety
We look forward to being part of the future of food safety and are here to help support your business in any way we can. Let us know how we can help!
How accurate are your product cooking instructions?
By Brian Schaefer, Technical Services Manager
Cooking instructions are considered an essential part of any new product development or existing product modification. Proper cooking instructions play a pivotal role in ensuring that a product meets food safety standards and maintains proper appearance, flavor, aroma & texture. There are three areas to consider when designing an effective cooking validation:
- Combination of time vs. temperature to meet food safety standards
- Method of temperature evaluation
- Selection of cooking equipment to be used
Combinations of Hold Time vs. Hold Temperature
Cooking validation relies on a combination of hold time and hold temperature to ensure a product meets food safety standards for human consumption. Hold times are defined as the amount of time that a product remains at the final temperature (hold temperature) after it has been removed from a heat source.2 Typically, when determining time vs. temperature combinations, an internal temperature of 165.2°F for 12 seconds is targeted to achieve a two log reduction of L. monocytogenes1 for ready-to-eat products and 165.2°F for 35 seconds in not ready-to-eat products. Since time vs. temperature combinations can vary, it is important to consider how the product will be consumed prior to determining the proper combination of hold temperature vs. hold time. For example, a single serve product with a hold temperature 158°F would require a 40 second hold time, however, shorter hold time could be more appropriate if the product may be consumed immediately after cooking. This scenario would require a higher hold temperature be achieved. Table 1 shows the hold times required to achieve 2, 4 or 6 log reduction depending on the hold temperature (calculated from ECFF, 20061).
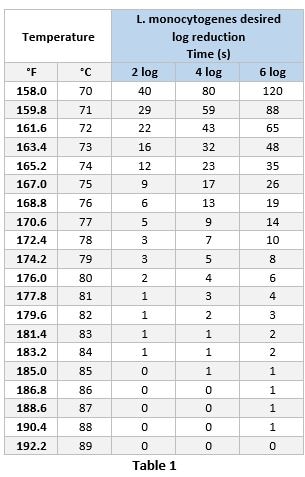
Method of Temperature Evaluation
Oftentimes, a product may not be uniformly heated during the cooking process; resulting in cold spots and undercooked areas in the product. Due to this, it is essential to identify cold spots and undercooked areas that may not reach the required temperature so that accurate cooking instructions can be developed. This can be done using anywhere from 1-10 probes simultaneously to measure the internal temperature of a product over at least a 2 minute interval. This temperature data is used to generate accurate post-cooking heating & cooling curves and ensures that proper cooking instructions are created.
Selection of Cooking Equipment
It is important to note that not all ovens or microwaves heat equally. When selecting a microwave, the label wattage does not always match the operating wattage of the unit. Verifying the operating wattage of the unit is essential to understanding the impact on the cooked product. For ovens, the power type (electric vs gas), size of the cabinet, trimming patterns while heating, and design (built-in vs. freestanding) all have an impact on cooking and can affect cook times. To account for these variations, multiple different types of equipment are selected and multiple replicates performed to ensure the cooking validation is robust.
If the desired cooking instructions are not sufficient, modifications can be attempted to achieve the required temperatures. Potential cooking instruction modifications could include:
- Instructions to stir product after cooking
- Instructions to rest product after cooking
- Additional cook time
- Cooking at different power/temperature levels
- Cooking the product covered or uncovered
- Flipping the product during cooking
Do you know how accurate your cooking instructions are for your products? Or have you recently changed one of your products and need to validate if your instructions are still correct? We can help validate that your cooking instructions ensure your products meet food safety standards. Please reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com for more information.
References:
1 )https://www.ecff.net/wp-content/uploads/2018/10/ECFF_Recommendations_2nd_ed_18_12_06.pdf
2) https://www.usda.gov/media/blog/2011/05/25/cooking-meat-check-new-recommended-temperatures
May is Food Allergen Awareness Month!
Food allergies are a significant safety and public health concern in the United States, with as many as 32 million Americans affected [2]. They are defined by an immune system response that ranges from mild symptoms to life threatening reactions; with even trace amounts of allergens triggering reactions in some individuals [1].
The FDA helps to mitigate this risk to Americans by enforcing the Food Allergen Labeling and Consumer Protection Act of 2004. This Act requires that food manufacturers clearly label products that contain ingredients from one of the major food allergens or contains any protein derived from these major food allergens [1].
While over 160 foods have been identified as causing allergic reactions, the following eight foods have been identified by the Act as causing 90% of food allergy reactions [1]:
- Peanuts
- Tree nuts
- Milk
- Egg
- Wheat
- Soy
- Fish
- Shellfish
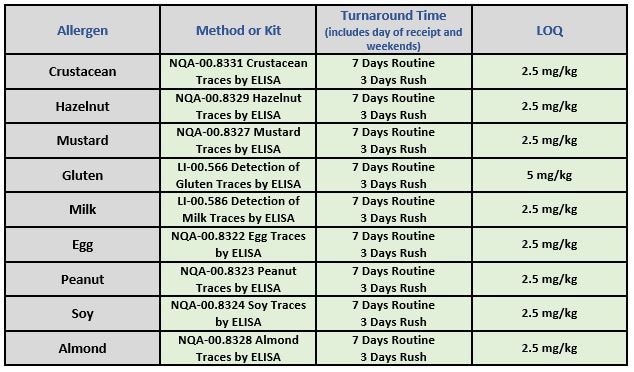
NQAC Dublin is committed to the safety of consumers and ensuring the integrity of your brand. We currently offer testing for the following common allergens:
- LI-00.566 – Detection of Gluten Traces by ELISA – Chem
- LI-00.586 – Detection of Milk Traces by ELISA – Chem
- LI-08.084 – Beta-Lactoglobulin by ELISA – Chem
- NQA-00.8322 – Egg Traces by ELISA – Chem
- NQA-00.8323 – Peanut Traces by ELISA – Chem
- NQA-00.8324 – Soy Traces by ELISA – Chem
- NQA-00.8327 – Mustard Traces by ELISA – Chem
- NQA-00.8328 – Almond Traces by ELISA – Chem
- NQA-00.8329 – Hazelnut Traces by ELISA – Chem
- NQA-00.8330 – Detection of Sesame Traces by ELISA – Chem
- NQA-00.8331 – Crustacean Traces by ELISA – Chem
- NQA-00.8332- Detection of Pecan Traces by ELISA – Chem
If you are interested in any of these allergen testing options above or an allergen not listed, reach out to our Customer Service Team at nqacdublincustomerservice@us.nestle.com for additional testing options!
References
[1] https://www.foodallergyawareness.org/food-allergy-and-anaphylaxis/food-allergy-basics/
[2] https://www.fda.gov/food/buy-store-serve-safe-food/what-you-need-know-about-food-allergies
What’s In Your Water?
By Gabriel Sanglay, Ph.D
With the addition of eight new microbiological methods for potable water testing to our portfolio, choosing the right tests might seem like a daunting task. In order to ensure safety and quality of your potable water supply or finished products, we offer a multitude of tests to meet your specific needs. Below is a description of tests and their provided information:
- Total Coliforms (quantitative)/Total Coliforms and E. coli by Colilert (qualitative): Coliform bacteria are defined as Gram-negative, nonspore forming rods that ferment lactose to produce acid and gas [1]. Coliforms can include genera such as Escherichia, Klebsiella, Hafnia, Enterobacter, Citrobacter, etc. These bacteria are commonly found in the environment or in the digestive tracts/feces of animals and humans. While most coliforms are not harmful to human health, they can act as indicators of the presence of pathogenic bacteria and/or fecal contamination [1, 2]. Per the EPA’s National Primary Drinking Water Regulations, the maximum contaminant level goal (MCLG) of coliforms is zero, while the maximum contaminant level (MCL) is no more than 5% total coliform positive samples within a month [2]. For facilities that test less than 40 samples per month, no more than one sample can be total coliform positive in a month [2]. Even though the EPA’s Total Coliform Rule only requires an absence/presence result per 100ml of potable water, quantitative data can provide information of the magnitude of contamination [3]. We offer both a quantitative membrane filtration that includes coliform confirmation, as well as a qualitative method that provides the absence/presence of total coliforms and E. coli.
- Heterotrophic Plate Count (HPC; Pour Plate at 35°C, Membrane Filtration at 35°C or 22°C): HPC, which is also known as standard plate count, is a procedure that measures the amount of live, culturable microorganisms in water [4]. HPC does not have any negative health effects [2]. We offer a standard pour plate method, or membrane filtration of 100ml at two different temperatures (the lower temperature method may improve recovery of stressed or fastidious bacteria) to monitor HPC in your water.
- Our other methods (Yeast and Mold Enumeration, Enumeration of Sulfite-Reducing Anaerobic Bacteria, Enumeration of aeruginosa by Rapid PA) are not required by EPA, but provide information on the cleanliness of the potable water supply, or quality issues with finished product.
- Fungi (which includes yeasts and molds) are present in the environment and can make their way into drinking water systems [5]. These organisms may be pathogenic, allergenic, or toxigenic and can be of particular concern to immunocompromised patient in hospitals [5]. They may also affect drinking water quality, affecting taste or odor in water sources or finished products [5].
- Sulfite-reducing anaerobic spore-forming bacteria (notably, Clostridium perfringens) can be resistant to disinfection processes, but their presence can serve as an indicator of protozoa, fecal contamination, or the effectiveness of filtration/disinfection processes [6].
- Pseudomonas aeruginosa can be an opportunistic bacterial pathogen, but rarely causes serious illness [6]. It is a common environmental organism, and its presence in potable water or finished products may affect taste, odor, and turbidity [6].
Please note, that while these are validated methods, they are not EPA certified nor is NQAC Dublin an EPA certified lab.
If potable water/finished product testing is of interest to you, NQAC Dublin can help. Below, you will find links to our technical datasheets for more information on our testing options. If you have further questions, please do not hesitate to reach out to our Customer Service team at NQAC Dublin at nqacdublincustomerservice@us.nestle.com.
Technical Datasheets
- ISO-4831:2006 – Detection & Enumeration of Coliforms – Most Probable Number Technique – Micro
- LI-10.112 – Yeasts and moulds enumeration – Micro
- LI-10.114 – Sulfite-Reducing Anaerobic Spores Enumeration – Micro
- LI-10.222 – Enumeration of P. aeruginosa by Rapid PA – Micro
- SMEWW 9215B – Heterotrophic Plate Count, Pour Plate – Micro
- SMEWW 9215D – Heterotrophic Plate Count, Membrane Filtration – Micro
References
[1] ScienceDirect. (2020). Coliform Bacteria. Retrieved from: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/coliform-bacteria
[2] Environmental Protection Agency. (2009, May). National Primary Drinking Water Regulations Complete Table. Retrieved from: https://www.epa.gov/sites/production/files/2016-06/documents/npwdr_complete_table.pdf
[3] Standard Methods for the Examination of Water and Wastewater, 23rd ed. (2017). Section 9222B – Standard Total Coliform Membrane Filter Procedure Using Endo Media. p. 9-82 to 9-88.
[4] Standard Methods for the Examination of Water and Wastewater, 23rd ed. (2017). Section 9215 – Heterotrophic Plate Count. p. 9-53 to 9-856.
[5] Hageskal, G. and others. (2009). Review – The Study of Fungi in Drinking Water. Retrieved from: https://www.sciencedirect.com/science/article/pii/S0953756208002414
[6] World Health Organization. (2017). Guidelines for Drinking-Water Quality, 4th ed. Chapter 11: Microbial Fact Sheets. pg. 231-306. Retrieved from: https://www.who.int/water_sanitation_health/publications/gdwq4-with-add1-chap11.pdf?ua=1
New & Improved Methods
We have expanded our portfolio of methods and made improvements to current methods to better serve our customers. Available as of March 23rd are the following exciting new methods and method updates:
- Fiber: We will replace AOAC 2009.01 Total Dietary Fiber in Foods to CODEX Definition Method AOAC 2017.16. The verified quantification limit will be 0.5g/100g and is applicable to foods, food ingredients and raw materials consistent with CODEX Definition fiber including naturally occurring, isolated, modified and synthetic polymers. This analysis meets the FDA’s definition for Dietary Fiber that can be declared on your Nutrition Facts panel and provides testing that better mirrors the human digestion process.
- Sugars: Our current sugars method will be replaced with a new method that will add Galactose as a reported component for Food Products and Finished Goods. This new sugars method (Carbohydrates NQA-52.003) will be applicable to juice concentrates and purees, food products and raw materials. The testing will be performed on an HPAEC-PAD platform with a detection limit of 0.05g/100g.
- Water: We will be implementing new methods in for potable water in Microbiology and for bottled and drinking water in Chemistry. Please note that while these chemistry methods are validated, they are not certified by the EPA, nor is NQAC Dublin an EPA certified lab.
If you have any questions regarding these new/updated methods, please feel free to reach out to nqacdublincustomerservice@us.nestle.com and we will be happy to answer any questions.

NQAC Dublin Business Update – Coronavirus (COVID-19)
As we deal with the Coronavirus (COVID-19) pandemic, ensuring continued food safety is extremely important. With this in mind, NQAC Dublin will continue to operate as “business as usual” to fully support the food industry while it works to provide food to consumers in this time of need.
Our top focus is to ensure we continue to meet customer needs while keeping our employees and communities safe. Please rest assured that we are taking all the necessary preventive measures to reduce risk, prevent exposure to illness, and prepare any back up plans that might be needed at our site.
We will provide updates if anything changes. If you have any questions or concerns, please reach out to us at nqacdublincustomerservice@us.nestle.com.
Food Safety and Microbiology Conference
NQAC Dublin Technical Services Manager, Brian Schaefer, appreciated the opportunity to share his knowledge and expertise in foreign body management at this year’s Food Safety and Microbiology Conference in San Antonio, TX. He enjoyed the opportunity to meet with other industry professionals and learn about the latest in Food Safety and Quality management.
We look forward to the opportunity to attend and participate in this event next year!
American Food Sure Summit
NQAC Dublin Director, Fabien Robert, enjoyed sharing his expertise as a presenter at the American Sure Food Summit in Chicago, IL on March 2-3. This event hosted food and beverage industry leaders from across North America and focused on the latest in food safety, quality, compliance, regulations, supply chain management, manufacturing and technology.
We appreciated the opportunity to present on Food Safety Microbiology Management, network with other industry professionals, and gain insight from other experts. We are looking forward to participating the Summit again next year!
The Importance of STEC Testing
By Gabriel Sanglay, Ph.D
You may have heard about Escherichia coli outbreaks/recalls in foods such as raw ground beef, spinach, romaine lettuce, ready-to-eat salad kits, packaged cookie dough, flour, and sprouts [1]. While a majority of E. coli are harmless, there are a few groups known to cause illness. Of these groups, the group of most concern are the Shiga-toxin producing E. coli (STEC).
STEC are E. coli that possess genes that code for either Shiga Toxin 1 or 2 (stx1, stx2), or both. Regardless of which Shiga toxin is present, all STEC serotypes are considered to be pathogenic [3]. Most STEC also possess an eae gene that codes for intimin, while not necessarily required for illness, but allows the bacteria to tightly attach to and cause lesions in the intestines [3,4]. The combination of stx and eae genes may correspond to an increasing severity of the illness in humans [4]. In the United States, O157:H7 has been the predominant STEC strain, but non-O157 serotypes have been implicated in more recent outbreaks (O26, O45, O103, O111, O121, and O145) [5].
When someone is infected with STEC, a person may experience symptoms of severe abdominal cramping and bloody diarrhea (also known as hemorrhagic colitis). In 3-7% of cases, complications may arise such as hemolytic uremic syndrome (results in destruction of blood platelets and kidney failure) or thrombotic thrombocytopenic purpurea (TTP; blood clotting in small blood vessels and low platelet counts). The mortality rate of people who develop further complications is approximately 3-5% [5].
A major reservoir for STEC are ruminants such as cattle, and the bacteria are shed through fecal matter. Infection can occur either via direct contact with these animals or fecal material, or from ingestion of contaminated food or water. Raw meat products may become contaminated by fecal material during slaughter, while fresh produce and grains become contaminated by either irrigation water, sewage runoff, improperly treated fertilizer, or direct contact with animals/fecal material [2].
If STEC is of concern in your raw materials, finished product, or environmental samples, NQAC Dublin provides a real-time PCR screening of the STEC virulence genes (stx1, stx2, eae) for your testing needs. If the screening indicates presumptive detection of the Shiga toxin genes, we also offer cultural and PCR-based confirmation analyses that will identify five of the STEC serogroups (O26, O103, O111, O145, and O157).
Below, you will find links to our technical datasheets for more information on our testing options.
ISO-TS-13136.2012 Detection of Shiga toxin-producing E. coli (STEC)
ISO-TS-13136.2012-B Confirmation of Shiga toxin-producing E. coli (STEC)
Interested in getting started or need more information? Reach out to NQAC Dublin for help at nqacdublincustomerservice@us.nestle.com.
[1] Centers for Disease Control and Prevention. (2020, February 26). List of Selected Multistate Foodborne Outbreak Investigations. Retrieved from cdc.gov: https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html
[2] Croxen, M.A. and others (2013, October). Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 26(4):822-880.
[3] Whitworth, J. (2020, January 31). EFSA: All STEC subtypes may cause severe illness. Retrieved from foodsafetynews.com: https://www.foodsafetynews.com/2020/01/efsa-all-stec-subtypes-may-cause-severe-illness/
[4] Boerlin, P. and others. (1999, March). Associations between Virulence Factors of Shiga Toxin-Producing Escherichia coli and Disease in Humans. J Clin. Microbiol. 37(3):497-503.
[5] Food and Drug Administration. (2012). Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd Edition. Enterohemorrhagic Escherichia coli. https://www.fda.gov/files/food/published/Bad-Bug-Book-2nd-Edition-%28PDF%29.pdf
Refrigerated Foods Association Conference
The 40th Annual Refrigerated Foods Association (RFA) Conference in Orlando, FL was a great success. This year’s conference featured a line-up of expert speakers discussing current issues in food safety and manufacturing, networking opportunities with industry leaders and a tabletop reception.
This year, NQAC Dublin had the opportunity to participate as an exhibitor and see the latest innovations in ingredients, equipment, packaging and services for the refrigerated foods industry. We had the opportunity to meet with several CEOs of Manufacturer member companies in a one-on-one session to learn about their company’s specific needs.
Our team thoroughly enjoyed this year’s conference and the opportunity to meet many industry leaders! We look forward to attending the 41st Annual RFA Conference in Scottsdale, Arizona next year!
USA CBD Expo
NQAC Dublin enjoyed meeting all of the attendees and exhibitors that we had a chance to speak with at the USA CBD Expo in Las Vegas last week! It is exciting to see all of the new and innovative products that are available and to be able to provide analytical services to a blossoming industry! If we didn’t have a chance to chat and you are interested in our services or analytical capabilities, please reach out to us at nqacdublincustomerservice@us.nestle.com, and we will be more than happy to assist you! We look forward to seeing you next year!
Is your Hemp or CBD product safe for consumers?
With the rising popularity of CBD and hemp-based goods, it is important to ensure that your consumers have confidence in the potency and safety of your products. Potency testing allows for the understanding of the exact cannabinoid levels in your product and is necessary for accurate product labeling. Contaminants testing, however, is essential in determining if your product is safe for consumption. Three chemical contaminants are of particular concern for hemp and CBD products.
- Heavy Metals and Trace Elements – Hemp plants are known to hyper-accumulate heavy metals (lead, mercury, arsenic, and cadmium) and other trace elements from the soil, fertilizers, and processing equipment that is used during production of concentrates and oils[1]. This results in a high likelihood that these products may have some level of elemental contamination and should be monitored through laboratory testing.
- Mycotoxins – Some fungi and mold species produce organic compounds and secondary metabolites known as mycotoxins. These mycotoxins, particularly aflatoxins (B1, B2, G1, and G2) and ochratoxin A, are capable of causing illness and death at a relatively small dose. Due to cultivation conditions, hemp plants are prone to mold and fungi growth and may be subjected to contamination at several points during harvest and processing[2]. Due to the significant health concerns resulting from mycotoxin contamination, hemp products should be routinely for mycotoxins by a qualified laboratory.
- Pesticides – Pesticides applied to the hemp plant, pesticide run-off from neighboring crops, and cross-contamination from municipal mosquito spraying or equipment bins may also be of safety concern. Regulatory requirements vary from state to state and can become concentrated during the extraction process[3]. For this reason, it is essential to test a product throughout the supply chain to ensure that pesticide residues that were below regulatory limits at one stage do not become an issue at another stage in the process.
From potency to contaminant testing, NQAC Dublin has an analytical method that will meet your hemp and CBD testing needs. Since our analyses are completed in our state of the art testing facilities by qualified chemists, you can feel confident that you are receiving accurate reliable results. Below, you will find links to our technical datasheets for more information on our testing options.
- Cannabinoids in Food and Raw Materials by LC-MS/MS
- Pesticide Residue Analysis by Electrospray LC-MS/MS
- Pesticide Residue Analysis by GC-MS/MS
- Analysis of Residues of Polar Pesticides in Foods of Plant Origin by LC-MS/MS
- Mycotoxins in Foodstuffs by LC-MS/MS
- Trace Elements Analysis by ICP-MS
Interested in getting started? Reach out to NQAC Dublin for help at nqacdublincustomerservice@us.nestle.com.
[1] Thomas, R. (2019, October 24). Beyond Potency: The Importance of Measuring Elemental Contaminants in Cannabis and Hemp. Retrieved from CannabisScienceTech.com: cannabissciencetech.com/article/beyond-potency-importance-measuring-elemental-contaminants-cannabis-and-hemp
[2] Atkins, P. (2019, December 6). Beyond Potency: Fungi, Mold, and Mycotoxins. Retrieved from CannabisScienceTech.com: cannabissciencetech.com/article/beyond-potency-fungi-mold-and-mycotoxins
[3] Jessup, I., & Goldman, S. (2019, December). Contaminates in CBD and ensuring consumer safety. New Food Magazine, pp. 60-63.
Microbial Identification Using MALDI-TOF
NQAC Dublin has implemented MALDI-TOF technology for microbial identification. This technology brings speed into the process and allows for rapid microbial identification in 1-5 days.
MALDI-TOF stands for Matrix-Assisted Laser Desorption Ionisation – Time of Flight and is a microbial identification system based on mass spectrometry of ribosomal proteins. This technology focuses on the ribosomal proteins of each microorganism, rather than on the expression variability that is normally seen in phenotypic methods that depend on growth of the microorganism and cultural conditions. This system is recognized as an official AOAC Method of Analysis and will be used during the confirmation and identification of Salmonella species, Cronobacter species, and other Gram-negative organisms (AOAC-OMA 2017.09) and the the confirmation and identification of Listeria monocytogenes, Listeria species, and other Gram-positive organisms.
Please reach out to Customer Service at nqacdublininfo@us.nestle.com if you have any questions about this new testing option!
New Method Announcement: STEC Detection and Confirmation
NQAC Dublin is excited to announce the introduction of two new methods to detect and confirm the presence of Shiga Toxin-Producing E. Coli (STEC):
- ISO-TS-13136.2012 – Detection of Shiga Toxin-Producing E. coli
- ISO-TS-13136.2012-B – Detection of Shiga Toxin-Producing E. coli
ISO-TS-13136.2012 is a real-time PCR screen that detects the major virulence genes for STEC: stx and eae. This new method will replace LI-00.799, allowing for cultural isolation and real-time PCR serogroup determination of the top 5 STECs – O26, O103, O111, O145, and O157.
What you need to know:
- The scope of these methods are applicable to products intended for human consumption and the feeding of animals, as well as environmental samples
- PCR screen of samples is currently validated at 25 gram replicates
- ISO-TS-13136.2012 will have a 7 day turnaround time
- Samples that screen Detected for STEC will automatically undergo confirmation testing at a 10 day turnaround time
Both methods will be coming soon! Please reach out to us at nqacdublincustomerservice@us.nestle.com for additional information on go-live or with any other questions you may have!
Method Update: LI-03.701 Vitamin Premix
As of November 13th, LI-03.701 Fat Soluble Vitamins for premixes will be updated to allow for billing by analyte. This will provide an upfront cost savings as well as allow for more flexible transitions between vitamins methods in the event that the sample submitted is not ideal for a specific method.
As always, please reach out to us at nqacdublincustomerservice@us.nestle.com with any questions you may have!
Chicago Section IFT Annual Suppliers’ Symposium & Expo
NQAC Dublin enjoyed meeting all of the attendees that we had a chance to speak to during the Suppliers’ Expo in Chicago on November 6th! If we didn’t have a chance to chat and you are interested in our services or analytical capabilities, please reach out to us at nqacdublincustomerservice@us.nestle.com, and we will be more than happy to assist you! We look forward to seeing you all at next year’s event!
New Method Announcement: Fat by Oracle
NQAC Dublin is excited to announce that we will be introducing a new fat testing method, going live on November 11th 2019, NQA-55.0001 Fat Determination by ORACLE.
CEM Oracle is a low-resolution NMR that offers a rapid and simple fat analysis in food. The products are dried using the traditional oven drying method and then analyzed on the ORACLE for fat content.
What you need to know:
- The ORACLE method is currently validated for sweetened condensed milk with a QL of 0.05 g/100g
- Testing will be offered on a 7 day routine and 5 day rush turnaround time
We are looking to further extend the scope of this method for future testing, please reach out to NQAC Dublin Customer Service (nqacdublincustomerservice@us.nestle.com) for additional matrices of interest.
New Offering: Nutritional Labeling
In May 2016, the FDA announced changes to labeling requirements that will require food and beverage manufacturers to update their nutrition fact label and associated claims.
These required updates include:
- Increased, bolded font for “calories” and “serving size”
- Inclusion of “added sugars” in grams and percent daily value
- Required inclusion of potassium and Vitamin D
Our new Nutrition Facts Panel package helps you meet the above requirements by providing you with:
- A full FDA compliant single column vertical panel in PDF format
- A full nutritional testing report with all supporting analytical data
Let us help you maintain compliance for your products and consumer confidence in your brand! Contact us today!
Coming Soon: 2020 PCQI Sessions 
With the successful roll out and completion of NQAC Dublin on-site PCQI training we are excited to be extending this service into 2020! Join us and become a Preventive Controls Qualified Individual.
Course Description:
The Current Good Manufacturing Practice, Hazard Analysis, and Risk-based Preventive Controls for Human Food regulation (referred to as the Preventive Controls for Human Food regulation) is intended to ensure safe manufacturing/processing, packing and holding of food products for human consumption in the United States. The regulation requires that certain activities must be completed by a “preventive controls qualified individual” who has “successfully completed training in the development and application of risk-based preventive controls”. This course, developed by the FSPCA, is the “standardized curriculum” recognized by FDA; successfully completing this course is one way to meet the requirements for a “preventive controls qualified individual.”
This course is taught by Lead Instructors trained by the FSPCA, who have been instructed in how to teach the FDA-recognized standardized curriculum.
Lead Instructor: Ben McPhail
Date :February 25th – February 27th, 2020
Location: 6625 Eiterman Road
Dublin, Ohio 43016
For additional information and signup coming soon for this course please visit https://www.nqacdublin.com/training-courses/.
Laboratory Shutdown – 12/26/2019
REMINDER: In addition to our 2019 holiday closures, NQAC Dublin will also be shutdown on Thursday, December 26th 2019.
On the day following the Christmas holiday the laboratory will be closed for maintenance. We kindly request that you please do not schedule deliveries for this day.
We will resume processing as normal on Friday, December 27th 2019.
For this and all future holiday closures (including 2020) please visit our website at https://www.nqacdublin.com/contact/
Food Allergen Testing – ELISA Methods
Undeclared food allergens can be very dangerous and are a major food safety concern. It is important to understand how food allergen methods work, how to choose the appropriate method, and how to interpret the results.
The primary method of detecting and quantifying food allergens is through enzyme-linked immunosorbent assays (ELISAs). Most ELISAs use antibodies to detect proteins from allergenic foods by using a sandwich format. Microwells are coated with an antibody and blocking agent. When the sample extract is added, any proteins present in the food will bind to the antibodies on the microwell. This process is then repeated with another antibody to form a sandwich. This antibody will have an enzyme attached which will cause a color change in the well, indicating the presence of a food allergen.
Most ELISA results are reported in ppm. The reported units are very important when interpreting these results, as the same sample analyzed by another method using different units will yield different results under similar conditions. It is also important to select the appropriate method for each circumstance. Take a milk allergen, for example. You should not choose a method that targets casein if the allergen is due to a whey protein isolate ingredient. Lastly, it is essential to remember that processes like fermentation and thermal processing can affect the quantification of allergens by ELISA.
NQAC Dublin currently offers gluten, milk, egg, peanut, soy, almond, mustard, crustacean, hazelnut, pecan, and sesame allergen testing by ELISA. Contact us at nqacdublincustomerservice@us.nestle.com to learn more.
Sources:
Downs, Melanie L., and Joseph L. Baumert. “Understanding Food Allergen ELISAs.” Food Quality & Safety Magazine, 2019, pp. 21–23.
Food Safety at Home
The CDC estimates that 1 in 6 Americans get sick, 128,000 people are hospitalized and 3,000 die from eating contaminated food every year. While some people are more at risk for foodborne illness than others, nobody is immune from the possibility of getting food poisoning.
At NQAC Dublin, we take food safety very seriously. We offer a variety of testing and services to help businesses keep the food they sell to their customers safe and nutritious. But that is not always enough. Consumers also play an important part in food safety.
We can all do things at home to protect ourselves and our families from foodborne illnesses. Here are some reminders of what we can do at home to help prevent illnesses from occurring. Food Safety Education Month is a good time to spread the word about food safety at home, but remember it is very important to take precautions anytime you are preparing and handling food.
Take a look below at some steps you can take at home to keep your families safe:
- WASH YOUR HANDS: Use soap and warm running water to wash your hands before handling, preparing or eating foods.
- WASH and CLEAN YOUR UTENSILS and PREP AREA: Begin with clean utensils, cookware, cutting boards and counter tops.
- AVOID CROSS-CONTAMINATION: Keep your foods separate. Always keep raw meats and eggs away from other foods-in your grocery carts, bags and refrigerator.
- NEVER RINSE RAW MEAT. It does more harm than good to rinse raw chicken. You are potentially spreading germs when spraying with water in your kitchen.
- PROPERLY STORE FOODS. Put any refrigerated or frozen foods away as soon as you get home from the grocery store. Keep your freezer and refrigerator at the proper temperatures.
- CLEAN YOUR VEGGIES AND FRUITS. Make sure you rinse well any fruit or vegetable even if you are not eating the skin/rinds.
- TAKE TEMPS. Use a thermometer to check that the foods you are cooking have reached the appropriate internal temperatures to kill bacteria.
- KEEP LEFTOVERS SAFE. Store them in the right size containers and place in the refrigerator within 2 hours. Also, toss any leftovers after 3-4 days.
- FOLLOW DIRECTIONS. It is very important to properly follow any directions on packages to ensure the foods have been cooked thoroughly. Food companies have taken special precautions to ensure that the cooking instructions on the back of the packaging ensure that if followed, the food will be cooked safely for your consumption.
We are here to serve as your top food safety resource for testing, shelf-life studies, environmental monitoring, cooking instructions validation and more. If you would like additional information on how we can support your business’ food safety needs, please reach out to nqacdublininfo@us.nestle.com today to discuss your needs.
Sources:
https://www.cdc.gov/foodsafety/education-month.html
http://www.fightbac.org/wp-content/uploads/2017/10/Food_Safety_Tips_Handout.pdf
AOAC 2019 Annual Meeting
This year’s AOAC Annual Meeting in Denver, Colorado was, again, a great success. NQAC Dublin enjoyed the opportunity to attend sessions, roundtables, and workshops as well as the chance to meet top scientists from around the globe. We are looking forward to attending next year’s meeting!
New Method Now Live!
We are excited to announce a new addition to our ever growing method portfolio! As of August 19th 2019, you are now able to request the analysis of Nicotinamide Riboside at NQAC Dublin.
What you Need to Know:
- This method will be applicable to complete nutrition clinical products
- Testing will be performed on a HILIC-MS/MS platform
- Detection limits will vary by matrix type:
- Liquid samples will be available at 20 mg/100g
- Powder samples will be available at 200 mg/1oog
- Turnaround time for results is 7 days, including day of receipt and weekends.
With a goal of seamless implementation for all of our methods, we encourage you to reach out to us regarding this new addition.
Contact our Customer Service group at nqacdublininfo@us.nestle.com and we would be more than happy to answer any questions you may have!
7th Year In a Row without Increasing Costs!
NQAC Dublin is happy to announce that for the 7th year in a row, we have not increased our prices while continuing to expand our analytical portfolio and service offerings! We understand how important it is to be cost effect while maintaining quality results for our customers.
Need a Copy of our ISO 17025 Accreditation?
Our latest accreditation certificates for both Microbiology and Chemistry analyses can always be found in the footer of this website. Scroll down to the bottom of any page and these are listed under “ACCREDITATION”.
Ten Must-Haves in a Laboratory Partner
NQAC Dublin’s Fabien Robert and Amy Bethel have contributed to the most recent issue of New Food Magazine: Guide to Testing.
Check out their Ten Must-Haves in a Laboratory Partner article here.
New Services at NQAC Dublin
In case you missed it, NQAC Dublin has added new services that we can offer your business as your trusted laboratory and food safety partner:
Environmental Monitoring
Is your facility in need of Environmental Monitoring Services? NQAC Dublin can assist! Our experienced team of quality experts will work with you to design a customized Environmental Monitoring Plan specific to you. Our non-biased approach results in an effective plan that indicates the true health of our factory environment. The support you receive will include a customized Environmental Monitoring Plan along with training resources, customized factory map with zoning, and support with data trending, corrective actions, and preventative controls. Please visit our Environmental Monitoring page for more information.
New Allergen Methods Available!
We are excited to announce the addition of two new allergen screens to our portfolio: Sesame and Pecan. Both have a 7 day routine and 3 day rush TAT. Please visit our Allergens page for the complete list of allergen tests offered.
Listeria Virulence Found to Vary Based on Food Type
A study performed by the Pasteur Institute found that the virulence of Listeria strains varies based on the type of food in which it is found despite long standing belief that food type had no effect on the virulence of the organism.
Hypervirulent strains of Listeria are often found associated with dairy products while hypovirulent strains are found to be associated more frequently with meat and fish products. The association of the different strains with different food types is due to the adaptability of the strains in the environments in which the food types are processed and their susceptibility to removal procedures in those environments.
Hypovirulent strains of Listeria are found to be more adapted to food processing environments and have developed more resistance to disinfectants; allowing them to adhere to and survive on surfaces. Hypervirulent strains are more adapted to the gut environment of hosts than to processing surfaces. Knowing the environments that these strains thrive in allows for the creation of a customized environmental monitoring program to curtail contamination events and will allow food manufacturers to put more effective contamination control measures in place.
NQAC Dublin now offers an environmental monitoring service where members of our team will visit your site and help you develop a monitoring plan that is specific to your needs. Click here to read more or sign up for a free consultation call!
NQAC Methods Earn AOAC and ISO Status
We are pleased to announce that our Chloride Method was approved by the AOAC Official Methods Board as Final Action. And our Total Amino Acids Method was First Action approved by AOAC.
Congratulations to two of our team members, Greg Jaudzems and Joseph Guthrie, for their contribution and hard work.
Thank you for visiting us at IFT19 in New Orleans!
Over 23,000 professionals from all areas of food science including ingredients, safety & quality, suppliers & buyers, packaging, R&D and manufacturing attended IFT 19! We enjoyed connecting with other food safety and quality professionals and learning more about the latest in our industry.
We are proud to be a part of this industry and look forward to working together and supporting your food safety testing needs in any way we can. If you would like more information on how we can support your business, contact us at nqacdublininfo@us.nestle.com.
We look forward to seeing you at IFT 20 in Chicago!
Environmental Monitoring
Is your facility in need of Environmental Monitoring Services? NQAC Dublin can assist! Our experienced team of quality experts will work with you to design a customer Environmental Monitoring Plan specific to you. Our non-biased approach results in an effective plan that indicates the true health of your factory environment.
The support that you will receive not only includes a customized Environmental Monitoring Plan but also:
- Training resources including: hands-on swabbing during factory visit, webinars, knowledge assessments, visual standards and videos for swabbing
- Customized factory map with zooming
- Support with data trending, corrective and preventative actions
- Excellent Customer Service seven days a week
Contact us today to discuss your Environmental Monitoring Need and learn how we can help: nqacdublininfo@us.nestle.com
Customer Day Reminder
Reminder that Quarter 2 Customer Day will take place on Tuesday, June 25 and there are still spots available! Any and all are invited to visit our laboratory in Dublin, Ohio. Look forward to future Customer Day announcements.
Tuesday, June 25th
- Experience the “Life of a Sample” with a tour of our facility and laboratories
- Learn more about Foreign Body Investigations and how NQAC Dublin can assist in a crisis situation
- Be the first to the introduced to the new Nutrition Facts Panel going live soon
- Have an opportunity to provide direct feedback through a Customer Service Focus Group
What you need to know:
- Each session will accommodate 15 attendees on a first come, first serve basis
- Lunch will be provided on site
- Travel and lodging will not be provided
If you or anyone you know is interested in attending Customer Day, please complete the attached form and email to: Samuel.Ventura@us.nestle.com. Also, feel free to contact Sam with any additional questions. We look forward to meeting you at Customer Day!
Nitrates and Nitrites in Food
For decades, nitrates and nitrites have been said to have adverse effects on human health. Because of these concerns the U.S. Department of Agriculture carefully controls the levels of nitrates and nitrites in meat product. However, some experts have found evidence that that concerns may not be entirely true.
Nitrates and nitrites are chemical compounds commonly used to prevent the growth of pathogenic microbes and provide desirable color and flavor in products such as bacon and sausage. However, the largest source of nitrates and nitrites in the human diet comes from plants, with vegetables making up approximately 85% of nitrate consumption in humans. This is thought to be because nitrates and nitrites a occurring naturally in soil and water.
Since the 1940s, nitrates and nitrites have been linked to cancer, thyroid issues, and other adverse effects on human health. Because of these concerns, the U.S. Department of Agriculture carefully controls the levels of nitrates and nitrites in meat products. However, some experts have found evidence that that concerns may not be entirely true. Some experts have found evidence that nitrate deficiencies may be a contributing factor in many chronic diseases since derivatives of nitrates play an integral role in human health; leading to reduced body weight, blood pressure, and related health problems.
Are you interested in determining the levels of nitrate and nitrite in your products? Reach out to us at: nqacdublininfo@us.nestle.com for more information.
Thank you for visiting us at the Food Safety Summit!
Thank you to everyone who stopped by our booth just to say hello or to learn more about our expanded services. We are proud to be a part of this industry and look forward to working together and supporting your food safety testing needs in any way we can. If you would like more information on how we can support your business or if you missed us at the show and want to learn about our services, contact us at nqacdublininfo@us.nestle.com
We look forward to seeing you at the Food Safety Summit 2020 in Chicago!
GMO Labelling Requirements: A deep dive into GMO detection using RTi PCR Methods
While international food trade is growing worldwide, the authorities in each country are setting more and more regulations to ensure food safety and transparency for consumers. Across the world Genetically Modified Organism (GMO) labelling is regulated in many countries. The United States will be joining the rest of world with GMO labelling regulation starting January 2020. This new regulation supports American consumers who are requesting more transparency and traceability on food-manufactured products.
With the expansion of GMO crops globally, new regulatory requirements and consumer demand for choice and transparency, increases the need for reliable GMO testing. This requires a solution that can screen, identify and quantify GMO crops to ensure a proper control to provide accurate results to meet regulation, labelling and consumer expectations.
Real-time polymerase chain reaction (PCR) is the most recognised tool for detection of GMO DNA sequences and recommended by Codex Alimentarious, ISO & EU reference laboratory for GM food and feed.
This webinar highlights the evolving world of GMO’s and a 3 step testing strategy using real-time PCR techniques to meet labelling regulations.
- Qualitative screening method to determine the potential for the sample to contain GMO.
- Additional testing to identify the GMO’s in the sample.
- For GMO’s identified, that have a tolerable limit Quantitative real-time PCR is utilized to determine the GMO % present.
The live webinar was on March 13 at New Foods Magazine but you can always watch it on demand here.
Don’t hesitate to reach out to us with any questions regarding GMO testing at nquacdublininfo@us.nestle.com.
Did You Know?
Customer Days Returns!
In 2018, NQAC Dublin began hosting Customer Days throughout the year to share our lab and create lasting connections with our customers. With the excellent feedback we received from those that attended, we are excited to announce this program will continue through 2019!
Our first Customer Day of the year will be Tuesday, March 26th. We look forward to meeting you!
Customer Day Sign UpThank you for your feedback!
At the end of 2018, we conducted our yearly NPS Survey and received helpful feedback from our customers. We consider all responses and this feedback helps us drive continuous improvement. Thank you for your feedback and participation in the survey.
If you have suggestions about how we can improve our services, please reach out to us at NQACDublinInfo@us.nestle.com.
New Allergen Methods Available!
With the introduction of allergen testing last year, NQAC Dublin has been continuously working to expand our capabilities and accommodate as many allergen screens as possible. We are very excited to announce the addition of two new allergen screens to our portfolio: Sesame and Pecan.
What you need to know:
- Sesame Allergen testing is validated for cookies, spices, sauces, coffee, infant formula, cereals, and environmental swabs.
- Pecan Allergen testing is validated for cake, cookies, chocolate, ice cream, and environmental swabs.
Please reach out to Customer Service at NQACDublinInfo@us.nestle.com with any questions about these new methods!
Refrigerated Foods Association Conference
The 39th Annual Refrigerated Foods Association (RFA) Conference in Tampa, FL was a great success. This year’s conference featured a line-up of expert speakers discussing current issues in food safety and manufacturing, networking opportunities with industry leaders and an exhibition reception.
This year, NQAC Dublin had the opportunity to participate as an exhibitor and see the latest innovations in ingredients, equipment, packaging and services for the refrigerated foods industry. We had the opportunity to meet with several CEOs of Manufacturer member companies in a one-on-one session to learn about their company’s specific needs.
Our team thoroughly enjoyed this year’s conference and the opportunity to meet many industry leaders! We look forward to attending the 40th Annual RFA Conference in Orlando, Florida next year!
In Food Safety, Preventative Action is Key!
Since 2012, food recalls and withdrawals have risen by 92.7% and have cost companies millions of dollars in lost revenue as well as negatively impacted their brand reputations. There is evidence, however, that when managed properly the negative impact to their brands was only temporary.
Reports indicate that a contributing factor to the increased number of food recalls is consumer awareness. Consumers today have unlimited access to information, have become more conscious about the quality of their food, and expect that companies should be held responsible and react to food safety issues swiftly.
A FoodLogiQ survey revealed that consumers expect that a recall should be resolved in as little as 1 to 2 days. Additionally, consumers expect to be kept up to date with relevant information throughout the food safety issue. When companies comply with the above consumer expectations, they can decrease the negative impact of the event to their brand reputation.
While it is important to act fast and take communication seriously during a food safety issue, a preventative approach is the best option.
At NQAC Dublin, we are dedicated to providing the highest quality analytical testing to help keep your consumers safe and your brand trusted.
Please contact us at nqacdublininfo@us.nestle.com for more information on how we can support your business.
American Food Sure Summit
NQAC Dublin Director, Fabien Robert, enjoyed sharing his expertise on Food Safety as a presenter and a panelist at the 7th Annual American Food Sure Summit. The event hosted industry leaders in Food & Beverage from across North America and focused on the latest in food safety, quality, compliance, regulation, food supply chain, manufacturing and technology.
We appreciated the opportunity to speak, network and gain insights from other industry experts and are looking forward to next year.
Protein Factor Conversion Tool Now Available
Are you ever in need of a different protein factor than what is reported? We recently added a Protein Factor Conversion tool to make it easy for you to do the conversion.
The Protein Factor Conversion tool is available with our other “Help Guides” on the Education & Resources page and can be found here: https://www.nqacdublin.com//education-and-resources/
By entering your reported nitrogen result into the designated cell applicable to your sample matrix, the tool will automatically convert this result with your needed factor. If you need help finding the tool or have questions on how to use it, please reach out and we will be happy to help: nqacdublininfo@us.nestle.com
Environmental Swabs – NEW 72 Hour Testing Option
We are very excited to begin offering a 72 Hour Environmental Swab option to provide more flexibility when shipping environmental monitoring samples to NQAC Dublin.
If you work in a food setting, chances are that you are familiar with environmental monitoring. You are also most likely familiar with the potential difficulties of ensuring your environmental swabs viability prior to testing. The current industry standard is to complete testing within 48 hours of environmental swabs being taken to ensure the viability of bacteria and avoid false negatives, as well as due to the potential for bacteria to increase or decrease over time. This short window of time can present issues. With this, NQAC Dublin worked to validate the use of HiCaP Neutralizing Broth to provide more flexibility with a 72 Hour option for Environmental Swabs. In order to take advantage of this new 72 hour testing option, specific materials will need to be purchased directly from World Bioproducts.
Contact us for specific items numbers to place your World Bioproducts order to take advantage of this new testing option: nqacdublininfo@us.nestle.com
Foreign Material? We Can Help!
Despite manufacturers taking as many precautions as possible, there are still instances of foreign bodies found in products by consumers. Several recent advances in technology can aid in the identification of these foreign substances, such as Fourier Transform- Infrared Spectroscopy (FT-IR) and X-ray Fluorescence (XRF) technologies. These techniques involve testing a sample of the foreign material found in a product as well as a suspected source material sample. Once testing is complete, the characterizations of these two materials are compared to determine the degree of similarity and identify the area of the manufacturing line where the foreign body was possibly introduced.
But what if the source of the material is unknown? One way to address this issue is by developing a materials characterization library for your manufacturing site. The library is created by mapping the materials used in manufacturing line parts and testing a representative sample of each of these materials. Then, if an instance of a foreign body within a product occurs, analysis of the sample can be completed and compared with the materials library for source identification.
Even if you do not have a foreign body currently that needs investigation, we can go ahead and get a head start on creating a library by line mapping your facility. Your materials characterization library will be ready if you do run into a foreign body issue at a later time. We can help you be prepared for the year ahead.
If line mapping and the creation of a materials characterization library are services that you are interested in, a member of our NQAC Dublin team would be more than happy to discuss your needs in greater detail and answer any questions.
Contact us today to learn more about these services: nqacdublininfo@us.nestle.com
In the news…
We are excited to share some recent news articles that we had the honor of contributing to in December.
New Food Magazine: Microbiology In-Depth Focus 2018, Quality Assured.
The Nestlé Quality Assurance Center in Dublin, Ohio, USA is regarded as setting industry standards when it comes to state-of-the-art facilities and technical sophistication. Here, Zone AMS NQAC head, Fabien Robert, tells New Food about the centre’s work and plans for the future.
Read the full article here.
Wall Street Journal: Probes of Foodborne Illness Outbreaks Multiply as Technology Improves.
NQAC Dublin Earns Ohio EPA’s Gold Environmental Stewardship Award
Over the past six years, NQAC has taken a number of steps to reduce our environmental impact.
“Through its recycling efforts and waste-reduction processes, Nestlé is well on its way to becoming a zero-waste facility,” Ohio EPA Director Butler said. “I am pleased to honor Nestlé with this award for environmental stewardship.”
Happy Holidays from NQAC Dublin

Updated FDA Guidance: Mandatory Food Recalls
In 2011, the Food Safety Modernization Act (FSMA) provided the Food and Drug Administration (FDA) with the authority to mandate food recalls. Prior to FSMA, recalls could only be initiated voluntarily by companies. This is still true today, the FDA typically relies on companies to voluntarily recall their products but can now use its authority to mandate a recall if necessary. They have only mandated a food recall once in the past seven years since they were given the power.
There were many questions related to this recall that was mandated by the FDA in April 2018. In response to the questions, the FDA recently released additional guidelines to help clarify when they plan to use their power to mandate a food recall. You can find their published guidance regarding recalls here:
And the FDA’s statement on the updated guidelines can be found here:
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm625061.htm
We Understand Lower Costs Help
NQAC Dublin’s commitment to customer satisfaction has allowed us to further improve our services while offering another year with no price increases!!
We are very happy to share that 2019 will be the sixth year in a row that we have not increased our prices for our testing services!
Allergen Cost Reduction & New Methods
We are continuously developing our Allergen Portfolio and are happy to announce that on December 10th 2018, we will be offering lower costs for these methods!
Additionally, we will now offer Crustacean and Hazelnut Allergens into our portfolio. Crustacean has been validated for finished food products, dairy products, cereal grains and environmental swabs. Hazelnut has been validated for cookies, chocolate bars, ice cream, cereals and environmental swabs.
Our NEW & EXCITING Allergen Portfolio is shown below:
New GMO Methods with Lower Costs
Our current qualitative GMO method (LI-00.038) will be replaced on December 10th 2018 with two NEW GMO methods that provide an array of benefits:
- Lower cost for Non-GM Samples!
- Increased ability to detect the widest range of GM-events!
- Identification of additional events which includes 5 GM-maize and 5 GM-soya!
The new qualitative “GMO screening by FAST Real-Time PCR” (LI-00.047) will detect if the most common GM-markers are present. This screen can detect greater than 95% of plant GMO’s being cultivated today! If your results are found as “Not Detected”, no further testing will be required.
If one or more of the GM markers are “Detected”, we will automatically analyze the sample using “GMO Identification by RTi-PCR” (LI-00.386). This identification method allows for the qualitative detection of 6 plant species and 6 GM-markers in addition to the specific identification of 21 GM-maize events and 14 GM-soya events.
The turnaround time for the initial screen and identification method will be 14 days or 7 days rush, which includes the day of receipt and weekends.
Sesame Next Allergen Possibly Up for Labeling Requirements
Consumers are calling for more and more transparency about what constitutes their food. For those with allergies, knowing what is in their food is extremely important so that those foods can be avoided. To help those with allergies not to limit the foods that they are eating out of fear of exposure, the FDA is looking into measures to require the declaration of Sesame on labels. Allergy labeling is currently only needed for milk, egg, fish, shellfish, tree nuts, peanuts, wheat, and soybeans.
NQAC Dublin has an extensive list of Allergen tests that we offer either at our laboratory or through a partnership with an approved third-party laboratory.
The available allergen methods include:
Almond, Cashew, Clam, Beta-lactoglobulin, Egg, Gluten, Hazelnut, Milk, Mustard, Peanut, Pecan, Sesame, Shellfish, Shrimp, Soy and Walnut.
New Sensory Test and Conversion Tool Now Available
A new method offering of Robinson Sensory Analysis is now available at our location as Taint Transfer from Packaging to Food. The analysis is aligned to ISO 13302 and EN 1230 standards.
The Robinson test determines whether a flexible packaging material (e.g. paper or board) contains substances, which could potentially be transmitted through the air space into your product (e.g. chocolate) and affect its taste.
A packaging sensory panel will be used to score the intensity of the submitted sample from 0 to 4, compared to a reference test portion with an intensity value of 0. The median score is then reported, with samples scoring 2.5 or higher considered to be of doubtful quality.
Our Vitamin conversion tool is also now available online! Do you ever need help converting your results into different units of measure? We recently added the Vitamin Unit of Measure Conversion Calculator to make this easier for you!
By simply entering your reported results into the designated cell on the spreadsheet, the tool will automatically convert the reported results to your desired unit of measure. If you have any questions on where to find the tool or how to use it, please reach out to Customer Service and we will be happy to help.
The Vitamin Unit of Measure Conversion Calculator is now available with our other “Help Guides” within the education and resources page on our website and can be found here: https://nqacdublin.com/education-and-resources/.
Foreign Body Investigation? We’ve Got you Covered!
While microbiological and chemical hazards are the most well-known food safety risks, physical hazards pose a potential threat and are also a source of consumer dissatisfaction. These physical hazards can be pieces of product that are not properly homogenized, pieces of processing equipment that have broken off, or substances introduced by the consumer on accident. An incident related to a foreign body can cause a company significant financial losses resulting from lawsuits and loss of consumer trust.
Recent advances in technology have aided in the identification of foreign bodies. Two techniques used for identification at NQAC Dublin are Fourier Transform- Infrared Spectroscopy (FT-IR) and X-ray Fluorescence (XRF). FT-IR takes advantage of the reaction of certain chemical bonds in organic molecules with specific wavelengths of light. This pairing of chemical bonds to wavelengths of light produces a characteristic peak that is used to identify the composition of a sample. This technology is most useful in applications where the particle in question is suspected to be an organic material.
XRF, on the other hand, is more advantageous when looking at a foreign body suspected to be a piece of glass or metal. This technology takes advantage of the fact that each element causes X-rays to exhibit different energies when irradiated with an electron beam. Each element emits its own, unique energy which allows for the identification of different materials. Different manufacturers of these materials use different levels of elements which allows the source of the contamination to be pinpointed.
Our team can also go a step further and do line mapping in your factory to create a library that will be useful in determining the source of the foreign body in question and also be used if there is ever a future investigation needed. If you are interested in our Foreign Body Investigation, please reach out to us at nqacdublininfo@us.nestle.com.
Columbus State Students Visit NQAC Dublin
NQAC Dublin was pleased to host 30 honor students from Columbus State Community College this fall. During their visit, the students enjoyed a tour of the laboratories and spent time with our Chemistry Manager (Becky Melick), Microbiology Manager (Brittany Pace) and Human Resources Manager (Joe Walter).
Our team enjoyed meeting the students and focused on educating them on potential careers in food science. Also, we wanted to introduce the many career paths and opportunities available within Nestlé globally, in the US, and locally within NQAC Dublin.
This visit and the information exchanged will potentially help students understand and choose a career path in food science and will also help ensure Nestlé Quality Assurance continues to attract talent that delivers the highest quality analytical results to all customers.
Thank you Columbus State for this opportunity!
The Impact of a Salmonella Outbreak
According to the Centers for Disease Control and Prevention, Salmonella causes approximately 1.2 million illnesses a year. The severity of these illnesses range from symptoms resolving without treatment within a few days to hospitalizations and, in extreme cases, death. Salmonella can infect anyone. Children under the age of 5, adults over the age of 65, and people with weakened immune systems are at the highest risk for severe illness.
So far this year there have been 12 reported Salmonella outbreaks. This is a significant increase from the amount of reported outbreaks in 2017. Salmonella is most commonly associated with raw or undercooked meat, eggs, and poultry. Many would be surprised to discover, that two of this year’s outbreaks are linked to breakfast cereal and pre-cut melon. The outbreaks linked to cereal and pre-cut melon have affected over 200 people in 38 states.
The impact of Salmonella outbreaks is not limited to public safety. To mitigate the food safety risk presented by these outbreaks, manufacturers often issue product recalls, which are likely to have financial and brand trust impacts as well.
Testing the ingredients and foods for potential food safety issues, such as Salmonella, other pathogens and physical hazards, are a large part of what we do here at NQAC Dublin.
Contact us at nqacdublininfo@us.Nestle.com for more information on how we can support your business.
New Method Available and Updated LOQ for Multi B Vitamins
New Method for the Analysis of Aminoglycosides!
We are proud to announce that the Analysis of Aminoglycosides in Food by LC-MS/MS is now available. This method analyzes for 14 aminoglycosides and is applicable to the following sample types:
- Milk-based products including milk fractions, infant formula, growing up-formula, adult formula, infant cereals, and baby foods
- Meat- and fish-based products including powdered, fresh, cooked; infant cereals, and baby foods
- Fatty matrices including animal fats, milk fats, and oil
- Egg-based products including white and whole egg powders
Providing the Most Accurate LOQ Possible
In our continuous effort to provide the most accurate results possible we are updating our Multi B Vitamin method Limit of Quantification to match the below stated ranges:
If you have any questions about the above offerings, please reach out to our Customer Service team for assistance!
National Food Safety Month
September is National Food Safety Month (NFSM) and gives us a great opportunity to highlight the importance of food safety and our commitment to quality.
Food safety is at the forefront of everything we do. We understand the responsibility associated with food safety testing and the importance of ensuring that the food, beverages, and ingredients tested in our facility are held to the highest standards.
What is the cost of food safety? Whatever the investment might be to create a culture of food safety within your business, it does not compare to the costs of a food product recall and the potential loss of trust from consumers. The Food Marketing Institute estimated that the average direct cost for a food recall in the US was $10 million and this number does not include indirect costs such as those associated with lawsuits, loss of sales, or damage to reputation.
Our commitment to quality and reliable results is embedded in our work and demonstrated daily by our staff, so that our customers can feel confident in the results they receive from NQAC Dublin. NFSM was created with a focus of increasing awareness of the importance of food safety through education. Check out our upcoming webinars, educational materials & resources page, to learn more about important food safety related topics.
EDUCATIONAL MATERIALS & RESOURCES
Sources
- “Create A Culture Of Food Safety.” National Restaurant Association, National Restaurant Association, 30 Aug. 2017, www.restaurant.org/Pressroom/Press-Releases/National-Food-Safety-Month- Highlights-How-To-Creat.
- Ostroff, Stephen. “The Costs of Foodborne Illness, Product Recalls Make the Case for Food Safety Investments.” Food Safety Magazine, Food Safety Magazine, 2018, www.foodsafetymagazine.com/magazine-archive1/junejuly-2018/the-costs-of-foodborne-illness-product-recalls-make-the-case-for-food-safety-investments/.
NQAC Dublin’s Expansive Testing Portfolio
Is there a test that you need but you do not see it offered in our already extensive testing portfolio? Just ask us!
We are continuously reviewing our method portfolio to meet customer requests and the changing market regulations. We take pride in listening to our customers and improving our services offered based on the feedback we receive. We strive to be the one stop shop for all your testing needs.
If you have any testing suggestions or questions, please contact us at nqacdublininfo@us.nestle.com. One of our team members would be more than happy to assist to you!
Packaging Materials and Food Safety
When most consumers think about food safety risks, their first thoughts are typically pathogens or unwanted chemical contaminants. And, yes, those are potential food safety risks, things we don’t typically want in the foods we eat and feed our families. Testing the ingredients and foods for these potential food safety issues are a large part of what we do here at NQAC Dublin.
But have you ever thought about the packaging materials that are meant to protect the foods you eat? Have you ever considered that those packaging materials could be impacting the safety and quality of the food you buy? No? Well, we have.
At NQAC Dublin, we have a portfolio of packaging tests used to identify potentially unsafe chemicals that could be coming into contact with the foods you eat. We perform packaging testing on an array of materials, including plastic, flex and cans. Our lab uses a variety of technologies to ensure the packaging that comes into contact with your food products are safe.
If you have questions about testing your packaging and the food products that packaging has come into contact with, reach out to us at: nqacdublininfo@us.nestle.com and one of our team members will provide additional information.
IAFP 2018
This year’s annual International Association for Food Protection (IAFP) was another great success. Every year, the IAFP hosts an event focused on the latest food safety issues and the science and solutions centered on the problems facing our industry.
Our team enjoyed networking and discussing pressing food safety issues with other professionals from all over the world. It’s always a great opportunity to connect with others in our industry and focus on new technologies that are available to help combat food safety risks. And once again, the agenda for the event did not disappoint. The presentations were led by renowned experts and covered a wide range of current food safety topics.
We are looking forward to not only attending IAFP 2019 in Louisville, Kentucky, but also participating as an exhibitor. Our team is very proud to be a part of such an important organization.
MCPD Studies
A collaboration between the FDA, SGS, Health Canada, and NQAC Dublin completed a comparison of analytical methodologies for the analysis of bound MCPD and Glycidol. This was presented by the FDA at the 2018 American Oil Chemists’ Society (AOCS) Annual Meeting in May.
Comparison of Analytical Methodologies for the Analysis of Bound MCPD and Glycidol in Edible Oils and Infant Formula
Process-induced chemical contaminants 3-monochloro-1,2-propanediol (3-MCPD) esters, 2-monochloro-1,3-propanediol (2-MCPD) esters, and glycidyl esters are formed in refined edible vegetable oils during the deodorization step of the refining process. As they are considered potentially carcinogenic and/or genotoxic, their presence in refined oils and foods may pose a potential health risk. For this reason, research efforts over the last several years have focused on developing methodology for the extraction and quantitation of these contaminants in oils, infant formula, and other complex food matrices in an effort to estimate levels of exposure. In addition, proposed EU regulations for bound 3-MPCD and glycidol concentrations in infant formula highlight the need for accurate analytical methodologies. Currently, there is no standard infant formula reference material containing known MCPD and glycidyl ester concentrations; therefore, verifying the accuracy of analytical methods has been difficult. This work compares a number of analytical methodologies for the analysis of bound 3-MCPD and glycidol in an attempt to confirm method performance.
These analytical methodologies include those used by the U.S. Food and Drug Administration, Health Canada, SGS Germany, and Nestlé for the quantitative analysis of bound 3-MPCD and glycidol in various samples of oils and infant formulas. In addition, data will be presented comparing the results of the use of direct and indirect detection methods with the infant formula extraction procedure developed by the FDA. A summary of the quantitative results obtained using each analytical method was presented.
In addition, our very own Greg Jaudzems will be presenting at the American Chemical Society (ACS) meeting in Boston on August 19th to talk about the influence of industrial process on 3-MCPD esters and Glycidyl esters in finished products.
Influence of industrial process on 3-MCPD esters and Glycidyl esters in finished products
The presence of fatty acid esters of monochloropropanediol is a food safety concern due to the carcinogenic properties of their fat-soluble components. 3-MCPD and glycidyl esters are process contaminants formed during industrial process. Their formation has been studied over the years and it is clear that the process conditions such as time, temperature and pH play a significant role. The presence of suitable precursor is also an important part. Research and studies have focused mainly on the analysis of raw material such as vegetable oils. Indeed, high concentrations of 3-MCPD and glycidyl esters have been found in this type of raw materials, mainly due to the deodorization step of the refining process. However, the influence of the process in finished products is relatively unknown due to the complexity of the process and number of ingredients involved.
In this study, infant formulae and their respective mixes of oils used for production have been analyzed. The objective is to relate 3-MCPD and glycidyl esters content from the raw material to the corresponding finish products. The results and effect of the process will be presented and discussed.
New Fiber Methods
The FDA has released new guidance on Dietary Fiber. The definition of Dietary Fiber has been expanded to ensure only fibers with beneficial effects on human health can be declared as Dietary Fiber on the food label.
You’ll need to ensure the testing methods you are using meet the challenges of the expanded definition. Our Dietary Fiber methodology is based on enzymatic-gravimetric-liquid chromatography and meets the requirements.
We offer the following AOAC methods:
2011.25 – Insoluble, Soluble and Total Dietary Fiber in Foods (CODEX Definition)
2009.01 – Total Dietary Fiber in Foods (CODEX Definition)
991.43 – Insoluble, Soluble and Total Dietary Fiber in Foods
These analyses meet the FDA’s definition for Dietary Fiber that can be declared on the Nutrition Facts label. We can help you select the correct Fiber Testing, contact nqacdublininfo@us.nestle.com and one of our team members will assist you.
ZERO Audit Findings!
NQAC Dublin is proud to announce three consecutive years of audit success. We received zero findings in our most recent external audit, which included:
-Environmental Management System – Certification to ISO 14002:2015
-Safety Management System – Continued Certification to OHSAS 18001:2007
-Quality Management System – Continued Certification to ISO 9001:2015
In 2017, we renewed our ISO 17025 Accreditation with zero deficiencies. Over 99% of our analyses are accredited under our ISO 17025 certification.
Our commitment to quality and reliable results is embedded in our work and demonstrated daily by our staff, so that you can feel confident in the results you receive.
Thank you for visiting us at IFT 18!
Over 23,000 professionals from all areas of food science including ingredients, safety & quality, suppliers & buyers, packaging, R&D and manufacturing attended IFT 18! It was great connecting with other food safety and quality professionals and learning more about the latest in our industry.
Thank you to everyone who stopped by our booth just to say hello or to learn more about our expanded services. We are proud to be a part of this industry and look forward to working together and supporting your food safety testing needs in any way we can. If you would like more information on how we can support your business or if you missed us at the show and want to learn about our services, contact us at nqacdublininfo@us.nestle.com.
We look forward to seeing you at IFT 19 in New Orleans!
Veterinary Drugs in Food Products
The Centers for Disease Control (CDC) estimates that more than 400,000 people become ill annually due to an infection caused by antibiotic-resistant foodborne bacteria. Antibiotic resistance is a growing concern for public health and food safety since an infection with an antibiotic resistant microorganism is typically more difficult to treat and may result in hospitalization or death.2
The use of antibiotics in agriculture is a contributor to the prevalence of antibiotic resistance. Livestock farmers use antibiotics in food-producing animals to promote growth and prevent the spread of illness between the animals. Even though these antibiotics may only be given in small doses in the food industry, this use contributes to the spread of antibiotic resistance.3 In fact, in the United States, there are more kilograms of antibiotics sold for food-producing animals than for human use.1
To assist with monitoring the level of veterinary drugs within your products, NQAC Dublin has recently implemented a new analytical method. This new method covers a wider range of compounds and product types while offering a quicker turnaround time for results.
If you would like to learn more about our testing options for veterinary drugs in food products, please contact us at nqacdublininfo@us.Nestle.com.
Sources
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats Report and Foodborne Germs. CDC.gov . [Online] April 14, 2014. https://www.cdc.gov/narms/resources/threats.html.
- —. Antibiotic Resistance, Food, and Food-Producing Animals . CDC.gov. [Online] July 24, 2017. https://www.cdc.gov/features/antibiotic-resistance-food/index.html.
- Barsky, Benjamin. Regulating the Antibiotic Resistance Crisis. theregreview.org. [Online] June 18, 2018. https://www.theregreview.org/2018/06/12/barsky-regulating-antibiotic-resistance/.
Food Fraud – Threats, Impacts & Combat – Fabien Robert at the Food Safety Supply Chain Conference
Fabien Robert, Director, Nestlé Quality Assurance Center (NQAC Dublin) spoke about food fraud at the Food Safety Supply Chain Conference in Rockville, MD. Food fraud is the alteration, concealment, dilution, mislabeling or counterfeiting of any food product at any point in the supply chain. The impact of fraudulent products in the food and beverage industry is substantial, just one instance can lead a business to bankruptcy and have dramatic consequences on public health. The cost of food fraud for the food industry exceeds $10 billion per year and impacts 10% of commercial products sold.
This symposium with attendees from across the food industry, including the FDA and food safety consulting companies, offered a great platform of discussion through multiple presentations. About 100 participants throughout the country reviewed the different aspects of food fraud and the corresponding best practices to fight it. The FDA explained in detail how Food Safety Modernization Act (FSMA) impacts this as well as the current actions being taken by authorities.
The presentations provided the perfect opportunity for conference participants and viewers to gain an understanding of FDA expectations and examples of food safety programs designed to protect a company from vulnerabilities in the supply chain. Food safety programs are very important for food manufacturers as food fraud negatively impacts everyone in the food supply chain.
At Nestlé, we take food fraud extremely seriously. We, like other multinational and national food companies, are engaged in the Global Food Safety Initiative (GFSI) program and have established a global food fraud prevention program dealing with economically motivated adulteration.
To keep food fraud risks under control it is critical to identify, review and set a management system in place. This system includes understanding the risk factors by building awareness internally and externally, promoting best practices, developing of prevention plans with vulnerability assessment, mitigation plan and continuous review. Transparency in the supply chain, supplier audits and raw material specifications, as well as robust analytical set-up, are additional elements to consider. Analytical techniques when implemented with statistical analysis help to prevent food fraud today and will continue to do so with the evolution of technology. These advancements, however, will never fully replace transparency and traceability throughout the supply chain.
At NQAC Dublin, we offer a portfolio of analyses to assist with identifying adulterated food products. Contact us today to learn more about our available authenticity testing to support your business needs.
Did you know undeclared allergens are the leading cause of food product recalls?
In a recent study, the US Department of Agriculture Economic Research Service investigated trends in food product recalls in the US from 2004 through 2013. The study found that between 2004 and 2008 the US averaged 304 recalls a year, while between 2009 and 2013 the average increased to 676 recalls per year. Based on the data, researchers were able to determine that improved pathogen and risk detection, as well as increased regulatory enforcement, were factors that contributed to this increase.
When researchers analyzed the recalls by type of risk, undeclared allergens were the leading cause of food recalls. Undeclared allergens accounted for 27.4 percent of all recall events. Due to the serious health risk to children caused by not accurately labeling allergens, legislation enacted in 2006 played a huge role in the increase of undeclared Allergen recalls. The Food Allergen Labeling and Consumer Protection Act (FALCPA) ensures that food manufacturers comply with practices to reduce or eliminate cross-contamination of allergens.
To help our customers remain compliant with federal regulations and to keep consumers safe, NQAC Dublin’s capabilities for allergen testing include:
Almond
Beta- Lactoglobulin (BLG)
Egg
Gluten
Milk Traces
Mustard
Peanut
Soy Traces
Contact us at nqacdublininfo@us.Nestle.com for more information on Allergen Testing.
Source: https://www.ers.usda.gov/webdocs/publications/88497/eib-191.pdf?v=43206
Food Quality & Safety Magazine: Nestle Expands Food Safety and Quality Support to Industry
NQAC Dublin announced at the Food Safety Summit 2018 that we opened our doors to other companies in the food industry.
Check out this article in Food Quality & Safety Magazine to learn more about our expanded services announcement:
Contact us at nqacdublininfo@us.Nestle.com for more information!
New Methods Now Live
AOAC 2009.01 Total Dietary Fiber in Foods
As of May 29, 2018, the analysis of Dietary Fiber by the CODEX Definition is available at NQAC Dublin as the official method AOAC 2009.01 Total Dietary Fiber in foods. This method provides an improved capability for Fiber analysis. The turnaround time is 14 days including the day of receipt and weekends. AOAC 2009.01 has been verified at our location with a quantitation limit of 0.5 g/100g. The method is applicable to foods, food ingredients and raw materials consistent with CODEX Definition 2009 including naturally occurring, isolated, modified and synthetic polymers. This analysis meets the FDA’s definition for Dietary Fiber declared on the Nutrition Facts Label.
LI-00.608 Vitamins A, E, D, K by UHPSFC-MS/MS
As of May 29, 2018 a new method for Vitamins A, E, D3, D2, K1 and K2 was implemented. This new method, LI-00.608 Vitamins A, E, D, K by UHPSFC-MS/MS, replaces our previous methods. The new method provides our customers with lower detection limits and utilizes supercritical fluid chromatography with a turnaround time of 7 days routine or 5 days rush including day of receipt and weekends.
The sample preparation for food products is identical to ISO 20633:2015 for the determination of Vitamins A and E, and AOAC 2016.05 for Vitamin D and AOAC 2015.09 for the analysis of Vitamin K1. The combined method allows streamlined bench work for gained efficiency. Reported units will be: Vitamin A as µg RE/100g, Vitamin E as µg TE/100g, Vitamin D and K as µg/100g.
AOAC INTERNATIONAL OMB Technical & Scientific Excellence Award Winner!
We are proud to announce that AOAC INTERNATIONAL has selected one of our own, Greg Jaudzems, for the Official Methods Board (OMB) Award for Achievement in Technical and Scientific Excellence for his study AOAC Official Method 2016.03 Chloride in Milk, Milk Powder, Whey Powder, Infant Formula, and Adult Nutritionals Potentiometric Titration Method First Action 2016. He has been invited to accept the award, along with his co-authors, at the upcoming AOAC INTERNATIONAL 132nd Annual Meeting and Exposition on Toronto, Canada on Monday, August 27, 2018.
Chloride in Milk, Milk Powder, Whey Powder, Infant Formula, and Adult Nutritionals by Potentiometric Titration Method: First Action 2016.03
The article discusses the AOAC International Inc. official method of potentiometric titration of chloride in milk, milk powder, and whey powder of infant formula, and adult nutritional formula. The methods are based on the standard performance requirements. Topics include the preparation of solutions, calculation of silver nitrate concentration for verification, and chemicals and reagents content.
30TH ANNUAL FOOD LABEL CONFERENCE!
NQAC Dublin attended the 30th Annual Food Label Conference hosted by Prime Label Consultants in Washington, DC on May 21-22. The conference is one of our favorite events which is always great for networking and keeping up to date on the very important regulations and labeling changes in our industry.
The conference has key speakers in the morning such as the FDA and USDA and allows the attendees to ask any questions they may have. In the afternoon, there are breakout sessions focused on various topics where you can learn in a classroom type atmosphere.
This year’s sessions included important topics like Trends in Clean Labeling, FDA Nutrition Labeling & Regulations Updates, USDA Update on Mandatory Bioengineered Food Labeling, Natural Labeling, Allergen Labeling and Nutrition Label Reform.
The 30th year celebration was a great year to attend, network, gain insights that you can put into practice and have your all questions answered! We learned so much and are very happy that we participated!

Thank you for visiting us at the Food Safety Summit!
Over 2,000 food safety professionals attended the 20th Annual Food Safety Summit in Rosemont, IL this year. It was great connecting with other food safety and quality professionals and an excellent opportunity to share the exciting news of our expanded services.


We look forward to seeing you again at the Food Safety Summit in May 2019!
Nutrition Labeling Date Finalized and GMO Rule Proposed
Labeling Compliance Deadline Concerning Nutrition Facts
In 2016, the FDA announced new requirements for what food manufacturers need to include on the Nutrition Facts labels of their products. This is in response to consumers wanting more information about the nutrition content of their food to make more informed choices.
Recently, the deadline for compliance was extended from July 2018 to January 1, 2020 for manufacturers with over $10 million in annual sales and for smaller manufacturers until January 1, 2021.
Original Label New Label

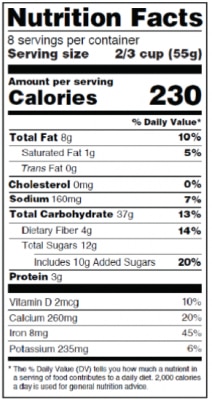
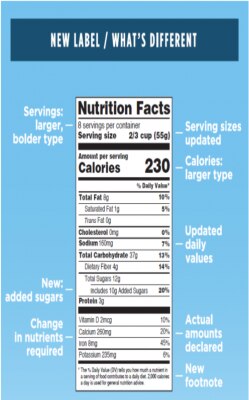
Proposed GMO Labeling Rule
Congress passed a law in 2016 establishing a mandate of the need to disclose the presence of food and ingredients from bioengineered (BE) foods. This was also in response to consumers wanting to make more informed decisions about the food they ate and what it was actually derived from. Manufacturers have several means through which they can disclose the inclusion of the BE foods: via text, icons containing “BE” or via a website.
In accordance with the new Nutrition Label deadline, large manufacturers with over $10 million in annual sales will have until January 1, 2020 and smaller manufacturers until January 1, 2021 to comply with this new labeling requirement on their product.


New Improvements to Laboratory Reports
In our ongoing Based on feedback from our customers, we have made changes to how the partial and final laboratory reports are received.
- The ability to opt out of Partial Reports is now an option.
- You now have the ability to customize the name of your report to make it easier to locate. To take advantage of this new feature, the Manual Microbiology Analysis Request Form (ARF), the Chemistry ARF and the Stability Protocol forms have new fields to insert a project description
-
- With this feature, the laboratory report will be received with a unique identifier followed by the project number. For example, the report will be received displaying Project Splash # 1816-0026. If this field is left blank, you will continue to receive reports in the same manner reading Final Laboratory report #1816-0026

-
-
- Any testing that is In Progress (IP) is now in bold, blue text for easier identification.
-
-
Method Portfolio Expansion
In our ongoing efforts to be our customers’ one stop for all their analytical needs, we are proud to announce the addition of three new methods to our portfolio of over 150 analytical methods. These methods have been brought internally to shorten the turnaround time for our customers.
The analyses of Chlorate and Perchlorate were made available April 23, 2018. With our commitment of service improvement, we brought this method in-house leading to a cost and turn-around time reduction with results available by seven days after receipt of sample. This method can be requested via LI-00.043.
Another method that was previously outsourced that has now been brought in-house beginning April 30, 2018 is the sensory analysis of packaging materials (Olfactory) under LI-80.017. With the addition of this method, the cost and turn-around time for our customers has been significantly reduced.
Based on the comments received from our customers to bring more allergen testing in-house, we are proud to announce the addition of Mustard Allergen testing to our portfolio on May 7, 2018. The launch of this method also reduces the turn-around time and cost.
Quality Assured: NQAC Dublin partners with the Ohio Department of Agriculture
NQAC Dublin is pleased to announce our partnership with the Ohio Department of Agriculture.
Check out this article in Ohio Farmer to learn more about our partnership:
http://www.ohiofarmer.com/technology/quality-assured-nestl
Contact us at nqacdublininfo@us.Nestle.com for more information!
Ohio Valley Institute of Food Technologists
Our team had the pleasure of supporting our local IFT section by volunteering at the Annual Golf Outing as well as the 2018 Suppliers’ Night Expo.

The Ohio Valley IFT Golf Outing took place at the Hamilton Elks Golf Course in Liberty Township, OH. Nearly 50 OVIFT members braved the wind and grey skies to enjoy networking and a round of 18 holes with fellow members.

The 2018 Suppliers’ Night Expo was at the beautiful Savannah Center in West Chester, OH. Over 80 vendors and 250 attendees enjoyed a day of networking.
NQAC Dublin is looking forward to attending the 2018 Great Lakes IFT Suppliers Expo on 5/2 as well as the 2018 Lake Erie IFT Expo on 5/22. If you are attending either event this month, let us know, we’d love to say “Hello!”.