Our Latest News
Dietary Fiber Testing: Getting the Numbers Right for Today’s Labels
Dietary fiber remains one of the most complex nutrients on a food label—not because it’s new, but because its definition and analytical methods have evolved. As fiber ingredients diversify and regulations expand, accurate testing is essential to support compliant labeling and credible nutrition claims.
What Counts as Dietary Fiber Today?
Dietary fiber definitions have moved well beyond the original focus on indigestible plant cell walls. Today, regulatory frameworks such as Codex and the FDA recognize dietary fiber as non‑digestible carbohydrate polymers (both naturally occurring and certain added or synthetic ingredients) provided they demonstrate a physiological benefit to human health.
A key challenge for manufacturers is that analytical methods cannot differentiate between fibers that meet regulatory definitions and those that do not. This makes method selection and documentation critical for accurate labeling.
Fiber Is a Nutrient, Not a Single Ingredient
Dietary fiber is a nutritional property, not one specific ingredient. It may include:
- Naturally occurring edible carbohydrate polymers
- Common fiber ingredients such as beta‑glucans, resistant starches, polydextrose, inulin, FOS, and GOS
- Grain‑based fibers from wheat, oats, barley, rye, and corn
These components vary in structure, solubility, and degree of polymerization (DP)—all of which influence how fiber is measured.
Why Soluble, Insoluble, and Molecular Weight Matter
Total Dietary Fiber (TDF) includes:
- Insoluble fiber, which passes through the digestive system largely intact
- Soluble fiber, which dissolves in water and is partially fermented
Fiber is also classified as:
- High molecular weight (HMW): DP >10
- Low molecular weight (LMW): DP <10 (primarily soluble fibers)
Modern labeling requirements may require quantification of both forms, making older gravimetric‑only methods insufficient in many cases.
Choosing the Right AOAC Method
AOAC methods have evolved to align with expanded fiber definitions:
- Earlier methods focused on HMW fiber only
- Interim methods added ingredient‑specific testing for LMW fibers
- Current methods, such as AOAC 2017.16 and AOAC 2022.01, quantify both HMW and LMW fiber—using gravimetric analysis and HPLC in a single workflow
Selecting the correct method depends on your formulation, fiber sources, and labeling needs.
How NQAC Dublin Can Help
NQAC Dublin offers several AOAC dietary fiber methods aligned with today’s regulatory expectations, including:
- Total dietary fiber testing
- Soluble and insoluble fiber differentiation
- Low‑molecular‑weight fiber inclusion where required
- Ingredient verification for fiber‑based formulations
AOAC 2017.16 – Rapid Total Dietary Fiber (CODEX Definition)
OM-AOAC-991.43_SDF_IDF – Total, Soluble, & Insoluble Dietary Fiber in Foods – Chem
OM-AOAC-991.43_TDF – Total Dietary Fiber in Foods- Chem
Whether you’re launching a new product, reformulating with added fibers, or validating nutrition labels, choosing the right dietary fiber method matters. Contact NQAC Dublin Customer Service to get started today!
Contact Us!
PCR vs. VIDAS: How to Choose the Right Detection Method for Salmonella and Listeria
Ensuring the microbiological safety of food products depends on rapid, accurate, and reliable pathogen detection. Whether screening for Listeria monocytogenes or Salmonella choosing the right method can dramatically influence turnaround times, result accuracy, and downstream confirmation work.
At NQAC Dublin, we rely on two primary categories of testing: molecular (PCR‑based) and immunological (VIDAS®). Each offers unique strengths—and understanding when to use which is key for food manufacturers, quality teams, and laboratory partners.
Molecular (PCR-based):
- LI-00.759 – Detection of Salmonella by IQ-Check® – Micro
- LI-00.708 – Detection of Listeria species by iQ-Check – Micro
- LI-00.708 – Detection of Listeria monocytogenes by iQ-Check – Micro
Immunological (VIDAS®):
- LI-00.705-FS – Detection of Listeria spp. by VIDAS® LPT – Micro
- LI-00.705 – Detection of Listeria spp. by VIDAS® LPT – Micro
- LI-00.742 – Salmonella VIDAS® Easy SLM – Micro
Why Pathogen Detection Method Choice Matters
Pathogens like Listeria and Salmonella are among the top microbiological hazards in the food industry. Proper method selection helps minimize false positives, ensures regulatory compliance, and supports timely product release.
The method you choose can impact:
- Speed of decision-making
- Ability to handle challenging matrices
- Specificity and sensitivity of results
- Risk of interference or inhibition
- Downstream confirmation workflows
Molecular Methods (PCR)
PCR methods target the DNA of pathogens, providing quick, sensitive detection. They are commonly used for high‑throughput operations and next‑day screening.
How PCR Works
PCR amplifies genetic material, enabling detection of even very low contamination levels. This makes it a gold standard for rapid, sensitive screening.
When PCR Excels
- Fastest turnaround for Salmonella
- High sensitivity
- High‑throughput settings
- Situations requiring strain differentiation
Considerations
PCR can be inhibited by matrix components—vitamins, pigments, spices—or by free DNA from environmental samples. Laboratories can often overcome inhibition using dilutions or enrichment modifiers such as Tween-20; if not, they transition to VIDAS.
Immunological Methods (VIDAS®)
VIDAS® assays detect pathogens through antigen‑antibody interactions, offering robust performance without the complexity of PCR instrumentation.
Where VIDAS Shines
- Highly resistant to PCR inhibitors
- Lower workflow complexity
- Reliable across diverse matrices
Specific Notes
- VIDAS Listeria methods are highly specific
- VIDAS Salmonella may show cross‑reactivity with Citrobacter
Head‑to‑Head Comparison
| Category | PCR (Molecular) | VIDAS (Immunological) |
| Turnaround Time | 28 hours for Listeria and 24 hours for Salmonella | 30 hours for Listeria; 50 hours for Salmonella |
| Sensitivity | Very high | Lower; requires longer enrichment |
| Specificity | High; risk of free DNA positives for Salmonella | High for Listeria; Salmonella cross‑reactivity possible |
| Matrix Compatibility | Can be inhibited | Highly robust |
| Workflow Complexity | Higher | Lower and more streamlined |
| Best Use Cases | Rapid testing; high throughput | Challenging matrices; PCR fallback |
How to Decide Which Method to Select:
Method selection involves evaluating three primary factors:
- Turnaround Time Needs: If speed is essential—especially for Listeria—PCR offers clear advantages.
- Matrix‑Related Inhibition Risks: Challenging matrices often push analysts toward recommending VIDAS from the start.
- Free DNA Concerns: Environmental swabs or samples from sanitized surfaces may trigger PCR presumptive positives for Salmonella that later confirm to be negative. For Listeria PCR testing, the workflow includes a “Free DNA” clean up step that minimizes this risk.
Choosing the right pathogen detection method for Salmonella and Listeria is essential for accurate, timely, and reliable results. PCR and VIDAS both play important roles in food safety testing—each suited to different matrices, turnaround time needs, and operational realities.
For food manufacturers and QA teams, the key is partnering with a laboratory that understands these nuances and can guide method selection based on science, experience, and real‑world performance.
Want to learn more about pathogen testing at NQAC Dublin?
Contact our Customer Service team to explore our complete pathogen testing portfolio or join us for our upcoming webinar – Pathogen Risks in Today’s Food Supply: Practical Strategies for Manufacturing & Quality Teams!
Register for Webinar!
AOAC Method Updates: Raising the Bar for Food Safety
We’re excited to share a major milestone in analytical excellence! Two Nestlé methods currently in use at NQAC Dublin have been officially endorsed as AOAC First Action Official Methods.
Why This Matters
AOAC officialization ensures the method delivers analytical results that meet the needs of regulatory use, food safety determination, and are accepted globally. In the United States, AOAC Official methods are recognized by the FDA for enforcement programs under 21 CFR § 2.19.
The Methods at a Glance:
PFAS Detection in Food (First Action 2025.10)1
In response to the AOAC Standard Method Performance Requirements (SMPR 2023.003) call for methods, Nestlé submitted our method developed for the quantitative determination of 57 per- and polyfluoroalkyl substances (PFAS) in food by liquid chromatography-tandem mass spectrometry. This method, which is used in routine at NQAC Dublin, was developed in accordance with the European Recommendation (EU 2022/1431) and Regulation (EU 2022/2388). During the summer of 2025, the method was unanimously approved by the AOAC Expert Review Panel for First Action status.
Pyrrolizidine and Tropane Alkaloid Analysis (First Action 2025.02)2
In 2023, Commission Regulation EU 2023/915 set forth maximum levels of pyrrolizidine and tropane alkaloids in a wide range of products. To ensure conformance and food safety, Nestlé developed and published “Pyrrolizidine and Tropane Alkaloids by LC-MS/MS” and implemented the method at NQAC Dublin. AOAC addressed the analytical need of the regulation with an advisory group panel and subsequent working group, publishing SMPR 2023.002 “Pyrrolizidine Alkaloids in Teas, Herbal Infusions, Dried Herbs, Seed Spices, Honey, and Botanical Dietary Supplements and Ingredients”. The Nestlé method was submitted as a candidate to the AOAC Expert Review Panel and granted First Action status in October 2025.
Ready to Take the Next Step?
Interested in implementing these AOAC-endorsed methods in your testing program? Contact our team today to learn how to get started or to request more information about the methods!
Contact Us!
- An LC-MS/MS method for the quantitative determination of 57 per- and polyfluoroalkyl substances at ng/kg levels in different food matrices, First Action 2025.10 Theurillat, X., Mujahid, C., Eriksen, B., Griffin, A., Savage, A., Delatour, T., & Mottier, P. (2023. Food Additives & Contaminants: Part A, 40(7), 862–877.
- Simultaneous and Quantitative Determination of Pyrrolizidine and Tropane Alkaloids in Food by LC-MS/MS, First Action 2025.02 Thomas Bessaire, Claudia Mujahid, Pascal Mottier, Ke Du, Andrew Savage, Xun Fu, Leland Hunter, Charmaine Teo, Wai-Chinn Chan, Journal of AOAC INTERNATIONAL, 2025;, qsaf097.
Celebrating Science in Action: NQAC Dublin’s Commitment to Safer Food
Every year on June 7th, the Food and Agriculture Organization of the United Nations and the World Health Organization work together to facilitate the observance of World Food Safety Day. The theme for 2025 is “Food Safety: Science in Action”; highlighting the essential role that science plays in ensuring that the food we eat is safe and nutritious.
Foodborne illness affects over 600 million people each year and poses a significant economic burden for low- and middle-income countries due to lost productivity and healthcare expenses1. Science is the foundation of food safety. Across every stage of the food supply chain, science has a part to play in keeping food safe.
At NQAC Dublin, we play an integral part in protecting consumers and keeping families healthy and safe.
Our scientists contribute to food safety through:
- Comprehensive Product Testing: Our laboratory boasts over 200 analytical and microbiological methods under one roof. This means that many of the food safety parameters you need to monitor in your products can be tested in one location. If we can’t cover your needs with our in-house methods, we will help you with finding a laboratory that can.
- Scientific expertise: Our chemists and microbiologists have significant experience in routine analysis using a wide range of analytical techniques. We are able to support your business regardless of whether you are monitoring chemical and microbiological contaminants or developing a new nutritional label for your products.
- Support for Manufacturing and Supply Chain: Our team can help you troubleshoot quality issues, as well as provide data-driven insights to improve your processes.
- Global Standards, Local Impact: We align with international food safety standards to ensure that your products are safe from farm to fork. We know that it is essential that your consumers can trust a product that they know and love. We help you meet this need, regardless of geographical location.
- Investment in Innovation: We are committed to expanding our capabilities, including instrumentation and automation, to stay ahead of emerging food safety challenges. Our laboratory boasts some of the most advanced instrumentation in the industry.
Your commitment to food safety is the foundation of consumer trust. Let’s work together to put “Science in Action” and make food safety a shared success. Contact us today to learn how we can support your food safety goals.
Contact Us!
Updated and New Method! Listeria spp. and Listeria monocytogenes
We are thrilled to present LI-00.708, a cutting-edge PCR method that leverages the Bio-Rad iQ-Check platform. This method has undergone independent validation to meet the ISO 16140 validation protocol and has received third-party certification by AFNOR. The LI-00.708 method represents a change, incorporating a new enrichment Listeria Special Broth II and an incubation temperature of 37°C, resulting in a reduced incubation time of 18-24 hours. Notably, LI-00.708 enables the lab to report L. monocytogenes as “Not Detected” or “Detected,” a capability that is not available with the current method LI-00.705. Additionally, the confirmation procedure provides more detailed information and is one day shorter than LI-00.705.
The current validation of matrix categories include environmental, composite foods, dairy products, and meats. We are in the process of evaluating two additional categories, namely vegetable and seafood, to ensure comprehensive coverage of all human foods. Furthermore, the lab has successfully verified multicomponent foods, confectionery, RTE/Ready to reheat meat, dairy products, egg products, flour, probiotic infant formula, and environmental.
Important Note: Not all matrices have been assessed for the LI-00.708 method, and certain matrices may be deemed unsuitable due to PCR inhibition or sample preparation issues.
LI-00.708 offers customers a range of testing options through the utilization of two distinct PCR kits. One kit is specifically designed to detect Listeria species, while the other targets Listeria mono. This flexibility empowers customers to select the most suitable kit based on their unique testing requirements.
Please contact our Customer Service team (nqacdublincustomerservice@us.nestle.com) if you have any questions about this new method or would like assistance in getting set up for testing!
Evaluation of Listeria Special Broth II (LSB II) for Recovery and Detection of Non-Stressed and Heat Stressed Cells
NQAC Dublin expert Gabriel Sanglay, PhD presented the findings of their study evaluating the recovery and detection of non-stressed and heat stressed cells using LSB II at this year’s annual meeting of the International Association of Food Protection (IAFP) in Long Beach, CA.
In this poster, Evaluation of Listeria Special Broth II (LSB II) for Recovery and Detection of Non-Stressed and Heat Stressed Listeria spp., the team evaluated the use of Bio-Rad Laboratories’ latest second-generation enrichment media that allows for detection of Listeria spp at 37°C at 18-24 hours post-incubation on the iQ-Check PCR platform. This enrichment media shows great potential for rapid detection of Listeria for routine testing.

Managing Listeria Risk in the Food Industry
The first half of 2024 has seen several recalls for products due to confirmed illness from Listeria or potential Listeria contamination. While the immediate concern is for the safety and health of consumers, recalls can also have serious impacts on manufacturers. Recalls often lead to significant financial losses, reputation damage, interruptions in operation, and increased regulatory scrutiny.
Listeria monocytogenes and other Listeria species are common environmental microorganisms that can contaminate foods during the manufacturing or packing process. Listeria monocytogenes is a pathogen can cause a mild, non-invasive gastroenteritis or a severe, invasive illness called listeriosis. Listeriosis is commonly associated with ready-to-eat (RTE) foods, especially those that have a pH greater than 4.4 and/or a water activity of greater than 0.92 (1).Furthermore, Listeria can survive and proliferate in harsh conditions longer than other non-spore forming bacteria of food safety concern and presents a major challenge to the food industry due to its persistence in food processing plants in harborage points (1).
Due to these challenges, having strong good manufacturing practices, sanitation procedures, and maintaining testing plans are essential to mitigating Listeria risk.
Manufacturing Practices/Sanitation
The FDA provides requirements for current good manufacturing practices (CGMP) and hazard analysis and risk-based preventive controls for human food in 21 CFR Part 117. Additional guidance on the control of Listeria monocytogenes in Ready-To-Eat Foods can be found in the Draft Guidance also available from the FDA.
If you are interested in becoming a preventive control qualified individual, NQAC Dublin can help! We offer PCQI sessions several times throughout the year. Check out our current available sessions below:
Sample Testing
The FDA recommends that in addition to implementing CGMP and sanitation procedures, both environmental and product testing to monitor the status of your sanitation procedures and process controls (1).
Once an environmental monitoring and sampling plan has been established, partnering with a qualified laboratory for sample analysis is essential to ensure accurate results. NQAC Dublin has extensive experience with both Listeria spp. and Listeria monocytogenes testing, with several methods available to best meet your testing needs.
- LI-00.705-FS – Detection of Listeria spp. by VIDAS® LPT – Micro
- LI-00.705 – Detection of Listeria spp. by VIDAS® LPT – Micro
- ISO-11290-1.2017 – Detection and Enumeration of Listeria monocytogenes – Micro
- ISO-11290-2.2017 – Detection and Enumeration of Listeria monocytogenes, Part 2: Enumeration Method – Micro
If you have any questions about our methods, or would like to get started with testing, please contact our Customer Service team for assistance.
Additionally, if you would like to learn more about microbiology in a laboratory or food processing facility, please watch our ON DEMAND webinar “Introduction to Microbiology: Principles and Applications to Food Safety”.
References:
Navigating PFAS Analysis in Food
By: Ashley Griffin, Senior Chemist
Per- and polyfluoroalkyl substances (PFAS) have quickly become a hot topic for both governing regulatory bodies as well as the mainstream media. The assessment for a better understanding of the presence of these compounds in the environment and the food chain prompted just as swift a response from laboratories to get food manufacturers analytical results for their products. The regulatory side of PFAS monitoring is also an ever-changing and evolving system. As more data is collected and analytical boundaries are being pushed, regulatory requirements are also emerging and updating constantly.
While the importance and the need for methods that can accurately quantify these compounds is quite apparent, PFAS analysis does come with its fair share of analytical challenges. These challenges, combined with the difficulties that come from the nature of these compounds as well as the stringent regulations being imposed on food manufacturers, produce a complex solution to screening for PFAS residues in food. NQAC Dublin has implemented two targeted LC-MS/MS methods: the first is a screening of 56 PFAS in various food commodities including baby food, infant formula, infant cereals, milk powders, meat, fish, eggs, fish oil/ vegetable oil, and coffee. The second is a limited scope method for the 4 regulated PFAS (namely PFOA, PFNA, PFOS, and PFHxS) in infant foods with LOQs ranging from 1-4 ng/kg.
Nestle will continue to monitor these changing regulations and guidelines when it comes to PFAS analysis and modify/ implement new PFAS methods if and when the time comes. It has become quite apparent that navigating these regulatory needs as well as the analytical challenges associated with testing for these compounds is an ongoing journey that will continue for years to come.
If you would like more information about our PFAS analysis, please view our on-demand webinar!
Sources:
acn_annual-report_2021-final.pdf (europa.eu)
The Rapid Alert System for Food and Feed – Annual Report 2020 (europa.eu)
New Methods and Method Updates!
Method Update! Determination of PAH by GC-MS/MS
We have recently updated our method to cover most matrices except for gummy type samples. This method will report out Benzo[a]anthracene, Benzo[a]pyrene, Chrysene, and Benzo[a]fluoranthene at a LOQ of 1 ppb.
New Method! Residual Intact Protein by LDS-PAGE
A brand-new analytical method option is available at our laboratory for intact protein measurement in whey-based moderately or partially hydrolyzed products. The procedure is based on gel electrophoresis.
Results delivered by this method are expressed as a binary result (detected/not detected at a screening target concentration of 200 mg/kg).
This method can also provide supplementary information related to the relative concentrations of residual intact proteins with a digitized image of the gel, but this is only meant to guide discussions and should not be reported as a result.
New Methods! Osmolality Measurements
We are pleased to announce the offering of two new analytical methods for Osmolality measurement in milk powders and similar products – Osmolality by freezing point depression (LI-08.066) and Osmolality by vapor pressure depression (LI-08.067).
These in-house procedures can be applied to most food products in liquid or reconstituted powder form, and has been specifically validated for reconstituted milk, nutritional powders, liquid milks, and liquid nutritional products.
Both tests require a minimum of 50 g sample for testing provide results on a variety of food types, but optimal results depend on the expected osmolality and the composition of the food.
The Freezing Point Depression method (LI-08.066) is applicable to low viscosity food products in liquid or reconstituted powder form. The optimal measurement range is 0-500 mOsm/kg. This method is recommended for measurement of product containing substantial amounts of volatiles (e.g. ethanol) or for osmolalites <100 mOsm/kg and products with low viscosities like water.
The Vapor Pressure Depression method (LI-08.067) is applicable to liquid or reconstituted powder form products that are viscous (about >2mPa*s) or have higher osmolality (>300 mOsm/kg) or on those containing particulate matter that can act as crystallization nuclei, which cannot be measured accurately by freezing point methodology.

This year’s World Food Safety Day theme is “Prepare for the Unexpected”. From producers to consumers, everyone must contribute to guarantee the safety of the food we consume. Despite our best efforts, there may be unforeseen circumstances where food safety is compromised. Food safety incidents can range from minor occurrences to major global crises. These can include power outages at home, food poisoning at local restaurants, voluntary recalls of contaminated products by manufacturers, outbreaks from imported goods, or natural disasters.
Our facility tests thousands of products to verify compliance with regulatory and food safety standards each year. It is an important responsibility as a food safety leader to help protect both consumers and food industry businesses. We hold all the products we test to the utmost safety and quality standards. Our commitment to quality and reliable results is embedded in our work and demonstrated daily by our staff. We prioritize a food safety culture every day so our customers can trust the results they receive from us.
World Food Safety Day is a great reminder that food safety is everyone’s responsibility. NQAC Dublin is here to support your business with all your food safety and quality assurance testing needs. Let’s partner together to ensure your customers feel confident in the foods they eat and feed their families.
Expert Support for your Food Safety Programs
A strong food safety program is critical to the success of your business and for the safety of your consumers. This program should be a “living document” that is revised and updated as your business grows and adapts to changes in the food industry. Each company is unique in how they implement their program, however most successful programs include:
- A team focused on food safety, including top management
- A strong HACCP plan
- A strong Food Safety Culture in facilities
If this process seems overwhelming, or you would like to ensure that your food safety program is complaint and robust, NQAC Dublin offers a wide range of support ranging from laboratory testing to PCQI training.
We provide accurate results, quick turnaround times and competitive pricing with industry-leading service to support your food safety and quality assurance needs.
If you have more questions or would like to discuss services and support options, please reach out to us at nqacdublincustomerservice@us.nestle.com we’d be happy to answer any questions you have.
REFERENCES:
(1) https://foodsafetytech.com/column/top-five-questions-when-building-a-comprehensive-food-safety-plan/
5 Questions about Shelf-Life Testing
In the food industry, conducting a shelf-life study is crucial to ensuring that products maintain quality, safety and nutritional value throughout intended shelf life. Below, we discuss how NQAC Dublin can provide valuable support in conducting shelf-life studies.
1. What is a shelf-life study?
A shelf-life study is conducted to determine the point at which a food product is no longer safe for consumption and when its quality, nutritional value and sensory attributes decline. These tests may involve microbiological, chemical and sensory analyses. The results of the study help establish the “best by” or expiration date for a product, indicating when it may no longer meet consumer expectations.
2. Is labeling with a “Best if Used By/Before” or expiration date required?
Food waste is a major issue in the U.S., and date labels often confuse consumers. The FDA provides guidance on writing date labels to help reduce confusion. Federal regulations don’t require product dating, except for infant formula. The “Use-By” date on infant formula is an expiration date and should not be exceeded. Date labels like “Best if Used By/Before” indicate quality, not safety.
3. Why/when do I need to conduct a shelf-life study?
Shelf-life studies are crucial in the food industry to maintain product quality, safety and nutritional value during the intended shelf life. Any alterations in raw material content or ratio, formulation, processing steps or packaging can influence the shelf-life of the product, making it necessary to assess its stability and determine a date label.
4. What if I need to launch a new product as quickly as possible?
Accelerated studies are used to quickly launch new products by storing them at higher temperatures and humidity levels, revealing potential sensory and chemical issues sooner. However, it is not recommended for microbiological testing due to potential alterations in microorganism growth. This data can estimate shelf life under normal conditions, but a real-time study is necessary to validate results from the accelerated study.
5. How can NQAC Dublin help with shelf-life studies?
Our experts can customize the study to meet your specific project requirements, whether you need a real-time or accelerated study. We have a range of controlled environments and analyses available to address the individual needs of your product. By partnering with us, you can obtain reliable data about your product’s shelf life throughout its intended storage period.
Reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com to get started!
The Importance of Cooking Instructions for Food Safety and Quality
Cooking instructions are vital for new product development and product modification. They are crucial in ensuring food safety, as well as maintaining the desired appearance, flavor, aroma, and texture of the product. A well-designed cooking validation study takes the combination of time/temperature to meet food safety standards, the method of temperature evaluation, and the cooking equipment to be used into consideration.
Hold Time and Hold Temperature
Cooking validation relies on a combination of hold time and hold temperature to ensure a product meets food safety standards for human consumption. A hold time is defined as the amount of time that a product remains at the final temperature, or hold temperature, after it has been removed from a heat source (1).
Typically, an internal temperature of 165.2°F for 12 seconds is targeted to achieve a two-log reduction of Listeria monocytogenes (2) for ready-to-eat products and 165.2°F for 35 seconds in not-ready-to-eat products. Since time vs. temperature combinations can vary, it is important to consider how the product will be consumed prior to determining the proper combination of hold temperature vs. hold time. For example, a single serve product with a hold temperature of 158°F would require a 40 second hold time. However, shorter hold time could be more appropriate if the product may be consumed immediately after cooking. This scenario would require a higher hold temperature be achieved.
Method of Temperature Evaluation
A product may not be uniformly heated during the cooking process, resulting in cold spots and undercooked areas in the product. Due to this, it is essential to identify cold spots and undercooked areas that may not reach the required temperature. This can be done using anywhere from 1-10 probes simultaneously to measure the internal temperature of a product over at least a 2-minute interval. This temperature data is used to generate accurate post-cooking heating & cooling curves and ensures that proper cooking instructions are created.
Selection of Cooking Equipment
It is important to note that not all ovens, microwaves or other appliances heat equally. For example, for microwaves, the label wattage does not always match the operating wattage of the unit. Verifying the operating wattage of the unit is essential to understanding the impact on the cooked product. For ovens, the power type, size of the cabinet, trimming patterns while heating, and design all have an impact on cooking and can affect cook times. To account for these variations, multiple different types of equipment are selected, and multiple replicates performed to ensure the cooking validation is robust.
Cooking Instruction Modification
If the desired cooking instructions are not sufficient, modifications can be attempted to achieve the required temperatures. Potential cooking instruction modifications could include:
- Instructions to stir product after cooking
- Instructions to rest product after cooking
- Additional cook time
- Cooking at different power/temperature levels
- Cooking the product covered or uncovered
- Flipping the product during cooking
For a more in-depth look at cooking validations and the science behind the creation of cooking instructions, please join us for our upcoming webinar.
Do you need help with validating your cooking instructions for a new product or a recently renovated product? Please reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com for more information.
References:
1 )https://www.ecff.net/wp-content/uploads/2018/10/ECFF_Recommendations_2nd_ed_18_12_06.pdf
2) https://www.usda.gov/media/blog/2011/05/25/cooking-meat-check-new-recommended-temperatures
Food Safety Culture: The Key to Ensuring Quality and Safety in Laboratory Testing
Food safety culture (FSC) has always been important for organizations, but it has gained even more significance in recent years with the FDA’s New Era of Smarter Food Safety program and the GFSI Benchmarking Requirements (Version 2020). A strong food safety culture is important not only for the manufacturer, but also for the laboratory that is performing your analytical testing.
What makes a strong food safety culture?
A strong food safety culture is a vital component of a robust Food Safety Management System (FSMS). It requires employees and leadership to have the right mindset and practice the correct behaviors consistently. When these behaviors are followed even when no one is watching, that is a true food safety culture. It is crucial to assess the maturity of FSC through internal and supplier FSMS audit programs to identify any challenges, barriers, or opportunities for improvement.
Why is food safety culture important for a laboratory?
Food safety testing plays a critical role in ensuring the safety and quality of food. While certifications, capabilities, and pricing are crucial factors when selecting a contract laboratory for food safety and quality testing, their FSC should also be evaluated. Laboratories with a strong FSC prioritize the understanding of the criticality of their work and encourage employees to adopt behaviors, practices, and values that prevent and detect issues that could impact the quality and safety of reported results.
How do we promote a food safety culture at NQAC Dublin?
At NQAC Dublin, promoting a food safety culture is one of our top priorities. We want to ensure that consumers feel confident in the food they eat and feed their families. Our commitment to quality is ingrained in everything we do and is demonstrated by our staff, the quality programs we have in place, and our leadership team. We provide educational and knowledge-sharing opportunities to our employees, encourage them to speak up if they notice anything concerning, and conduct cross-functional root cause investigations to address any problems that may arise.
How can NQAC Dublin help your company meet their food safety testing needs?
Our laboratory in Dublin, Ohio is the largest and most advanced testing facility in the Nestle network. With a large analytical portfolio and a range of services, our laboratory teams have the expertise to support your business and troubleshoot any issues you may encounter.
Please reach out to our customer service team today to get started with our team of experts!

Understanding Shelf-Life Testing and Its Importance in Food Products
When it comes to food products, ensuring their quality, safety, and nutritional value is of utmost importance. One key aspect of this is determining the shelf life of a product. Shelf-life testing plays a crucial role in assessing the period when a food product maintains its optimal quality, nutritional content, and safety for consumption.
What is Shelf-Life Testing?
Shelf-life testing involves a series of microbiological, chemical, and sensory tests conducted to determine the point at which a food product is no longer safe for consumption and when its quality, nutritional value, and sensory attributes decline. It helps establish the expiration date or “best by” date for a product, indicating when it may no longer meet consumer expectations.
Consumers expect food products to be safe, nutritious, and of high quality. Shelf-life testing ensures that products meet these expectations throughout their intended shelf life. While the FDA does not require a “best by” date for most food products, many retailers demand shelf-life studies to ensure compliance with consumer expectations and avoid selling expired products. Shelf-life testing helps manufacturers maintain consistent product quality by identifying potential issues that may arise during storage and distribution.
What Can Impact Shelf-Life?
Over the shelf life of a product, certain changes may occur that can affect its nutritional value. Several factors can impact the shelf life of a food product. These factors include:
- Moisture: The presence of moisture can promote microbial growth and accelerate spoilage, reducing the shelf life of a product.
- Temperature: Higher temperatures can speed up chemical reactions and microbial growth, leading to faster deterioration of the product.
- Oxygen: Exposure to oxygen can cause oxidation, leading to changes in flavor, color, and nutrient degradation.
- Light: Exposure to light, especially ultraviolet (UV) light, can cause photochemical reactions that degrade the quality and nutritional value of the product.
- Time: The longer a product is stored, the more likely it is to experience quality degradation and nutrient loss.
- Product Formulation: The ingredients and formulation of a product can influence its stability and susceptibility to spoilage.
- Processing Method: The processing method used, such as heat treatment or packaging techniques, can impact the shelf life of a product.
Shelf-life testing is a vital process in the food industry, ensuring that products maintain their quality, safety, and nutritional value throughout the intended shelf life. By considering the factors that influence shelf life and conducting comprehensive testing, manufacturers and retailers can provide consumers with safe, nutritious, and high-quality food products. Ultimately, shelf-life testing plays a crucial role in maintaining consumer satisfaction, regulatory compliance, and brand reputation in the competitive food market.
Testing Options at NQAC Dublin
Our laboratory team can support you in providing data about your product through its shelf life. Each shelf-life study can be customized to meet your project requirements. Below are a few of the potential options available for your study.
Controlled Environments
- Ambient Environment
- Temperature and Humidity Controlled
- Accelerated, Refrigerated, and Freezer Environments
- Limited Light Conditions
Analysis
- Microbial
- Probiotic
- Nutritional
Additional Testing
- Water Activity
- Moisture
- pH
- Headspace Oxygen
| Condition | Temperature/ Humidity |
| Accelerated | 40C/75%RH |
| 30C/75%RH | |
| 30C/65%RH | |
| Ambient | 25C/60%RH |
| Refrigerated | 4C |
| 6C | |
| 8C | |
| 10C | |
| Freezer | -15 C |
| -20 C | |
| -25 C |
If you are interested in conducting a shelf-life study for your product, please reach out to our Customer Service team today to get started!
References:
Shelf-life Testing – IFT.org
Fu, Bin & Mi, Creek & Labuza, Theodore. (2000). Shelf Life Testing: Procedures and Prediction Methods for Frozen Foods.
Kilcast, D., & Subramaniam, P. (2000). Introduction. In D. Kilcast, & P. Subramaniam, The stability and shelf-life of food (pp. 1-19). Cambridge: Woodhead Publishing Limited .
ON DEMAND: PFAS Summit: A Scientific Series
AJ Savage, expert chemist at NQAC Dublin, recently presented on the sources of PFAS contamination and the analytical impacts at the PFAS Summit sponsored by SCIEX.
Also presenting at this Summit, was Xanthippe Theurillat, Specialist R&D at Nestlé Switzerland. During her presentation, she discusses PFAS at ng/kg levels in food.
This summit is now available on demand! Please visit the below link to register and join the discussion as industry experts explore how these “forever chemicals” have influenced our food testing and research.

If you need assistance with testing for PFAS in your materials, our team can help! Please reach out to our customer service team today to get started with our team of experts!
Foreign Body Management: Strategies and Techniques for Food Safety
“Foreign Bodies” (also called Foreign Materials or Foreign Objects) refer to any material found within food or packaging that a consumer does not expect to find.
Foreign bodies can be physical, chemical, or biological in nature and often render the food unfit for human consumption. These materials can enter the chain of production at any point during processing and shipment.
In a 2020 review completed by TraceGains, it was found that out of the 363 recalls that were issued in 2020, 90 of those were due to foreign material contamination.
This trend appears to be continuing moving into 2024, with several high-profile recalls being issued related to the presence of steel, bone, rocks, and plastic.
In a legislative update published in Quality Assurance Magazine, David Acheson discusses the ways in which manufacturers can help drive down recalls due to foreign objects.
In this update, he advises that since foreign material introduction can occur anywhere during the manufacturing and distribution process, it critical that businesses focus on prevention with their suppliers and within their facilities.
Supplier Management
The supply chain can introduce risk for foreign materials to both your process and to your finished product.
Creating a robust Supplier Management program that includes understanding your supplier’s upstream processes as well as their control procedures can aid in preventing foreign material inclusion in your products. (1)
As part of your program, you should include specifications for suppliers, as well as conduct routine reviews of supplier programs to ensure compliance.
These programs are essential since screening equipment at the processor’s plant, such as magnets, metal detectors, and X-ray machines have limitations that can result in certain foreign materials not being detected or removed. Foreign materials that may not be detected include biological material such as insect parts, hair, or wood and different kinds of plastics and cardboard. (2)
Facility Management
Once the material arrives at the plant, detecting equipment can be helpful in screening incoming material as well as outgoing product for foreign material. Detection equipment such as X-rays and metal detectors require that all personnel using the equipment are adequately trained and the equipment is properly calibrated and validated. (3)
Detection, however, should not be the only process in place to prevent foreign material from entering product. Since failing equipment can often be the source of risk, a control program that includes preventive maintenance and inspection should also be in place. This will allow the facility to define the control measures and intervention that might be needed at any point in the line. A good sanitation program is also effective at removing foreign material contamination and should be used in addition to preventive maintenance and other control measures. (1)
Finally, it is critical that your employee training program includes foreign object risk as part of the overall food safety program for the company. All employees should be aware of the importance of the foreign material control program and understand the risk to food safety and consumer satisfaction. (1)
Foreign Material Identification
If a foreign object is found, either during in-plant inspection or by a consumer, it can be difficult to pinpoint the origin of the material.
Identification of the foreign body is frequently needed to assist in determining the source so the hazard can be removed, or consumer findings can be validated.
There are several techniques that can be used to aid in identification including:
- Microscopy
- X-Ray Fluorescence (XRF)
- Fourier Transform Infra-Red Spectroscopy (FTIR)
The methodology that is best suited to the sample often depends on a variety of factors, including the suspected composition of the object. Each of these techniques also has limitations depending on the material being analyzed.
For a more in-depth look at these technologies and some useful case studies, please view our on-demand webinar. Our expert will review each of the techniques, as well as discuss some of the limitations of each of the methodologies.
If you need assistance in the identification of a foreign material, or with setting up a foreign material prevention program, our team can help! Please reach out to our customer service team today to get started with our team of experts!
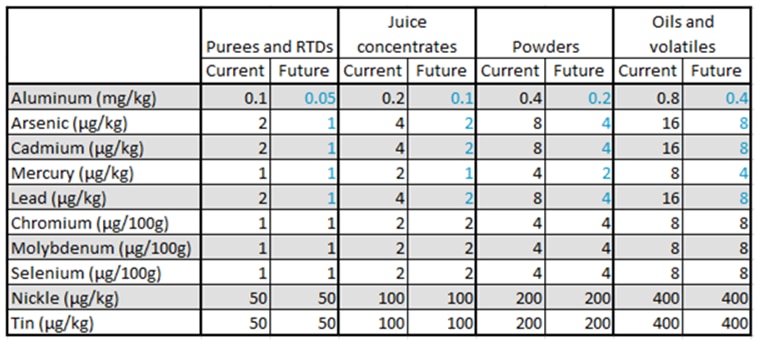
Understanding Dietary Fiber: Types, Analysis Methods, and Labeling Considerations
By: Ann Jobko, Chemist
Dietary fiber is defined by the FDA as naturally occurring fibers that are intrinsic and intact in plants, as well as added isolated or synthetic non-digestible soluble and insoluble carbohydrates with beneficial physiological effects to human health. For consumers, this means that the term dietary fiber refers to any carbohydrate that is not broken down by the body’s own enzymes in the small intestine.
Forms of Dietary Fiber
Total dietary fiber can be broken down into two forms: soluble and insoluble fiber. Soluble fiber dissolves in water and provides some calories. Insoluble fiber does not dissolve in water, so it passes through the gastrointestinal tract relatively intact and does not provide calories. Insoluble and soluble fiber are combined to give the total dietary fiber content in foods.
In addition to soluble and insoluble, fibers are also defined by their degree of polymerization. High molecular weight dietary fiber (HMWDF) is defined as non-digestible carbohydrates with a degree of polymerization greater than 10 (DP>10). Some examples of HMWDF include cellulose, pectin, β-Glucan and Galactomannan. While low molecular weight dietary fiber (LMWDF) is defined as carbohydrate polymers with a degree of polymerization between 3 and 9 (3≤DP≤9). Some examples of LMWDF are Fructooligosaccharides (FOS), Galactooligosaccharides (GOS), polydextrose and resistant maltodextrins.
Analytical Methods for Dietary Fiber
At NQAC Dublin, we run 3 methods to determine fiber content; these include AOAC 991.43, AOAC 2017.16 and AOAC 2011.25.
AOAC 991.43 is run to determine only high molecular weight fiber. This method can be used for products that do not contain a source of low molecular weight fiber. There can be an underestimation of the dietary fiber amount because most resistant starch and all non-digestible oligosaccharides are not included in the total fiber result. If the product does not contain resistant starch or low molecular weight fiber; AOAC 991.43 would be the cheaper and more efficient option to choose.
AOAC 2017.16 can be used to determine both HMWDF and LMWDF. This method is the official validated method that can accurately measure total dietary fiber and is our default method for nutritional labeling of fiber. This method changes the enzyme concentration and digestion times to improve the recovery of RS2, RS4 and FOS (fructooligosaccharides) without any of the overestimation issues seen in AOAC 991.43.
AOAC 2011.25 is a modified version of AOAC 2017.16. It can further break down the HMWDF into soluble and insoluble portions and gives the LMWDF amount. Depending on labeling requirements, individual soluble and insoluble fiber determinations may be required. If this is the case for the product being analyzed, AOAC 2011.25 would be the test to choose.
To choose the best fiber method for the product, it is essential to know the source of the fiber. All sources of fiber must be FDA approved for them to be claimed on the label. If the product contains fiber that is not FDA approved, the non-approved fiber percentage must be manually subtracted from the claim. No fiber method can separate approved and nonapproved fiber sources. If there are questions regarding specific ingredients or the most applicable method for products, AOAC 2017.16 can be used as a default or reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com for guidance.
If you would like to learn more about Nutritional Testing and how fiber is analyzed and calculated, please view our webinar with Food Safety Magazine.
Accurate Nutritional Labeling: Regulatory Requirements and Analytical Testing at NQAC Dublin
By: Jacob Snider, Chemist
Accurate nutritional labelling is a regulatory requirement for food products in nearly all markets. Here at NQAC Dublin we have an analytical profile that allows us to determine the content of nearly all important human nutrients accurately and precisely in a wide variety of foods. Below is an overview of the general regulatory requirements for the nutrition facts panel, and a more specific look into how our analytical portfolio can help you obtain accurate, reliable data to confirm your label claims.
Analytical testing can provide a level of confidence above and beyond any calculation from database values of raw ingredients. Numerous factors influence the eventual nutritional content of a finished food product that can be very difficult to determine by calculation. Raw materials vary in nutritional value due to processing variables, growing conditions, crop cultivars and storage conditions. Processing these materials into finished products introduces additional unknowns due to the interaction of heat, light and pH among many other factors that affect the stability and solubility of various nutrients.
Per FDA regulations in the United States, all food products must generally be labelled with a nutrition facts panel (NFP) detailing the serving size in both weight and a common household measurement, as well as the content of the following nutrients:
- Calories
- Total Fat
- Saturated fat
- Trans fat
- Cholesterol
- Sodium
- Total carbohydrates
- Dietary fiber
- Total sugars
- Added sugars
- Protein
- Vitamin D
- Calcium
- Iron
- Potassium
In addition to these nutrients, certain claims and additions to the product can require additional labelling. In general, if a product is specifically fortified by the addition of a nutrient, then that nutrient must also be declared. A manufacturer may also add any nutrient with an established Reference Daily Intake (RDI) if the content is accurately reported.
NUTRIENT CONTENT CLAIMS
If a product’s labelling includes any information that meets the criteria of a “nutrient content claim” it may require that nutrient to be included in the NFP. This is defined as a claim on a product that characterizes the level of a nutrient in the food. This includes statements such as “low fat” or “high in soluble fiber” or “good source of calcium.” These claims have specific definitions that products must meet, and generally require additional information on the NFP to support the claims. This can also include compounds which do not have established RDIs and thus would not be able to be included on the NFP but may be claimed elsewhere on the package. This information must still be accurate and thus analytical testing of any such claims may be prudent to be able to provide evidence of these claims.
FORMATTING AND ROUNDING REPORTED VALUES
In addition to the regulations regarding what nutrients must be included on the NFP, there are numerous rules regarding the proper method of formatting and rounding the reported values. E.g. calories must be rounded to the nearest five if below fifty per serving, or to the nearest ten above fifty per serving. Fats must be reported to the nearest half gram if below five grams, nearest whole gram if above five, and as zero if the total is less than a half gram per serving. These rules can be tricky, and they differ between analytes.
ANALYTICAL METHODS FOR NUTRIENT CONTENT
At NQAC Dublin, the analytes in the standard NFP panel are analyzed by the following methodologies.
Calories and Carbohydrates by Calculation
Using values known as Atwater factors we multiply the number of grams of fats, carbohydrates and protein per serving by the appropriate factor to determine the energy content (calories) of the sample.
Similarly, carbohydrates are also determined by calculation using the amount of protein, ash, moisture, and fat are measured by the analyses for those macronutrients. The percentage of the sample that is not accounted for by the analytically determined constituents is assumed to be carbohydrates. Nearly all common food matrices are made up of a combination of these compounds and because carbohydrates are the most difficult class to analyze, this technique is an industry standard practice for total carbohydrate determination.
Total Fat, Trans Fat, Saturated Fat and Cholesterol
Total fat, trans fat and saturated fat are determined by our fatty acid profile method. This method reacts the sample with a base undergoing a reaction known as saponification which converts all the fats present to their constituent fatty acids. The fatty acids are then analyzed by gas chromatography and the total fat as triglycerides is calculated from these results. The method Is able to determine all the different types of fatty acids present and it can thus also determine the trans and saturated fats.
Cholesterol is determined using the same sample extract but analyzed on a separate instrument in our laboratory.
Sodium, Iron, Calcium, and Potassium
Our method for sodium, iron, calcium, and potassium analysis digests the sample at a very high pressure and temperature with nitric acid in a microwave digestor. The resulting solution containing only extremely simple compounds and ions is analyze on an inductively coupled plasma atomic emission spectroscopy instrument. Different atoms emit characteristic wavelengths of light when they are excited by the high energy of the torch and this signal can be used to quantify the amount of many different minerals present.
Dietary Fiber
Dietary fiber can be determined through several methods, however for the NFP testing we utilize AOAC 2017.16. This method takes the sample through a series of enzymatic digestions and washing steps to remove fat solubles compounds and digest and remove all the non-fiber carbohydrates from the sample. The final extract is analyzed by high-pressure liquid chromatography to determine the amount of fiber present. The method can determine both the total and soluble fiber. For regulatory purposes it is important to ensure that all fiber-like compounds in the product are approved fiber sources as the method is not able to determine the difference between approved and non-approved fiber sources.
Total Sugars and Added Sugars
Total sugars are determined using an HPAEC-PAD system. This method dissolves the sample and analyzes it for all the common mono- and disaccharides via ion chromatography.
The added sugars value must be provided by the manufacturer for inclusion on the label. Unfortunately, there are no common quantitative methods that can distinguish sugars native to the product versus those added from other sources. Added sugars are defined as any sugar added to the product from concentrated sugar sources including fruit juice concentrates, any pure sugars such as sucrose or dextrose, beet sugar, honey, agave, and several other natural and artificial sources.
Protein
Protein is determined by the Kjeldahl method. In this method the sample is digested in the presence of a mixture of sulfuric acid and several other chemicals which eventually results in the conversion of all organically bound nitrogen to ammonia. The ammonia is then collected by distillation and its amount measured by titration. The amount of nitrogen present in the ammonia is correlated to a protein value using an appropriate value known as a protein factor.
Due to different protein amino acid compositions different sample types may have a different amount of protein present given the same amount of nitrogen. It is important to ensure that an appropriate protein factor is applied. For most food samples the generic value we use is appropriate, but in cases of deviation from expected values ensuring that the factor applied is accurate for your sample type is an important first step.
Vitamin D
Vitamin D, which consists of both D2 and D3 is analyzed in our laboratory using ultra-high-pressure supercritical fluid chromatography. This technique allows for easy and accurate measurement of many fat-soluble compounds. D2 is the form of vitamin D generated by fungi and can be used to fortify foods and retain their vegan status. D3 is the mammalian form of vitamin D and is often extracted from lanolin, a waxy substance derived from sheep wool.
CHALLENGES IN ACCURATE VITAMIN LABELLING
Vitamin A
One analyte that can pose difficulties with accurate labelling is vitamin A. There are two major categories of compounds with vitamin A activity, carotenoids and retinols, and they possess different levels of activity and require different methods to analyze. Here at NQAC Dublin we measure retinol species and carotenoids using different methods. The typical unit used to express the vitamin A activity of a food is the “microgram retinol activity equivalent”, or µgRAE. The conversion from carotenoids to µgRAE is complicated due to the different factors for the different carotenoids. The FDA also requires a different conversion to be used for carotenoids derived from supplementation and from food sources.
Vitamin E
Another analyte that has unique difficulties is vitamin E. Vitamin E is not one compound but instead a class of compounds broken up into two main categories of tocopherols and tocotrienols. Each category has alpha- beta- gamma- and delta- variants. Each of those variants has 8 stereoisomers, compounds with the same chemical composition but arranged in a different shape, of which only some exhibit vitamin E activity. Luckily, alpha-tocopherol contributes by far most of the vitamin E activity in most common food matrices.
It is also necessary to know the stereochemistry of these compounds to be able to determine their vitamin E activity, however there are no commonly performed assays that allow the separation of the stereoisomers. The “R-R-R“ stereoisomer is the only one that occurs in nature, while synthetic sources are generally an equal mixture of all eight isomers, of which only about half exhibit E activity. Thus, to determine the vitamin E activity manufacturers must use recordkeeping to determine the amount of synthetic versus naturally occurring vitamin E species in their product and apply the conversion factors supplied by the FDA to determine the actual vitamin E activity present in the food product.
NUTRITION FACTS PANEL TESTING AT NQAC DUBLIN
Accurate nutritional labeling is a crucial requirement for food products in various markets. Here at NQAC Dublin we provide a testing suite for all nutrients that must be included in the NFP. This test list provides the measured content of all the basic required label nutrients. To assist in this calculation, please provide the following information with your submission:
- Serving size in grams
- Serving size in a household measure (cups, tablespoons, pieces etc.)
- Servings per container
- Added sugars
With this information we can generate a compliant label using our analytical results that follows regulations regarding format, rounding and accuracy.
If you would like to learn more about Nutritional Testing and the applicable methodologies, please view our webinar with Food Safety Magazine.
If you have any questions regarding testing or would like to get started, please reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com.
Understanding PFAS and Ethylene Oxide: What You Need to Know For Testing
Ethylene Oxide
By: Heather Rice, Chemist
Ethylene oxide (EO), is a colorless flammable gas used in the food industry as a fumigate for dry food commodities. A small molecule with a strained ring structure, EO can penetrate and spread quicky throughout a product and is incredibly effective at destroying microorganisms. However, EO has been classified as a toxic substance by several establishments including the World Health Organization (WHO). Due to its hazardous properties, ethylene oxide has been banned as a pesticide in the European Union (EU) since 1991.
NQAC Dublin offers Ethylene Oxide analysis as part of our contaminants method portfolio to help meet your food safety needs. Due to its high volatility, EO is converted to 2-Chloroethanol (2-CE) which is then extracted using a QuEChERS based procedure. The resulting supernatant is then analyzed by GC-MS/MS. Results are expressed as total Ethylene Oxide with a quantitation limit of 0.01mg/kg. We offer testing on several different commodity groups, including ice cream, herbs, spices, thickeners, vegetables, sauces, cereals, protein-based samples, maltodextrin, food supplements and inorganic salts, flavoring and coloring.
As regulatory requirements governing EO usage continue to evolve, NQAC Dublin will continue to monitor regulations and guidelines and work to ensure we continue to meet the needs of the food industry.
Per- and polyfluoroalkyl substances (PFAS)
By: Ashley Griffin, Chemist
Per- and polyfluoroalkyl substances (PFAS) have quickly become a hot topic for both governing regulatory bodies as well as the mainstream media. The assessment for a better understanding of the presence of these compounds in the environment and the food chain prompted just as swift a response from laboratories to get food manufacturers analytical results for their products. The regulatory side of PFAS monitoring is also an ever-changing and evolving system. As more data is collected and analytical boundaries are being pushed, regulatory requirements are also emerging and updating constantly.
While the importance and the need for methods that can accurately quantify these compounds is quite apparent, PFAS analysis does come with its fair share of analytical challenges. These challenges, combined with the difficulties that come from the nature of these compounds as well as the stringent regulations being imposed on food manufacturers, produce a complex solution to screening for PFAS residues in food. NQAC Dublin has implemented a targeted LC-MS/MS method for the screening of 55 PFAS in various food commodities including baby food, infant formula, infant cereals, milk powders, meat, fish, eggs, fish oil/ vegetable oil, and coffee.
Nestle will continue to monitor these changing regulations and guidelines when it comes to PFAS analysis and modify/ implement new PFAS methods if and when the time comes. It has become quite apparent that navigating these regulatory needs as well as the analytical challenges associated with testing for these compounds is an ongoing journey that will continue for years to come.
Sources:
acn_annual-report_2021-final.pdf (europa.eu)
The Rapid Alert System for Food and Feed – Annual Report 2020 (europa.eu)
Mycotoxin Sample Size
A Key Component in Accurate Testing
The mycotoxin test procedure is a three-step process that includes sampling, sample preparation, and analysis. Each of these steps contributes to the overall uncertainty of the test procedure.
It is difficult to obtain an accurate estimation of the mycotoxin concentration in a bulk lot1. One way to help reduce the amount of uncertainty in the test procedure is to ensure that the sample is of adequate mass and from adequate number of points throughout the lot. Since mycotoxins are distributed heterogeneously throughout a lot, using a reduced sample weight or too few samples represent two of the most frequent errors that result in an overall increase in result uncertainty2.
A representative sample can be created by collecting small incremental samples from several randomly chosen areas of the material lot. These smaller samples can then be combined to create the large single aggregate sample that is submitted for analysis. For commodities that have a large particle size (nuts/grains) a larger sample size of up to 10 pounds is recommended. Commodities that are more homogenous in nature with a smaller particle size (flour) can use a smaller total sample size. Once the laboratory receives the representative sample for testing, the full sample amount sent is homogenized prior to testing. This ensures uniform distribution of mycotoxins throughout the sample portion.
There are several resources available that can help you in developing your sample plan:
NQAC Dublin offers several robust methods for Mycotoxin detection that can help you meet your food safety goals. Please reference the Technical Datasheets linked below for more information about each method:
- LI-00.027 – Tropane Alkaloids by LC-MS/MS – Chem
- LI-00.056 – Alternaria Toxins by LC-MS/MS – Chem
- LI-00.219 – Patulin by LC-MSMS – Chem
- LI-00.185 – Mycotoxins in Foodstuffs by LC-MS/MS – Chem
References
- Whitaker, Thomas. (2006). Sampling Foods for Mycotoxins. Food additives and contaminants. 23. 50-61. 10.1080/02652030500241587.
- Cheli, Federica & Campagnoli, Anna & Pinotti, Luciano & Eleonora, Fusi & Dell’Orto, Vittorio. (2010). Sampling feed for mycotoxins: Acquiring knowledge from food. Italian Journal of Animal Science. 8. 10.4081/ijas.2009.5.
Price Adjustment Coming in 2023
NQAC Dublin would like to thank you for your continued support and partnership in ensuring the delivery of safe food to consumers!
For many years now, our location was able to avoid raising prices to help our customers. However, we have all been affected by the challenging economic conditions this past year and with inflation reaching extreme levels we are no longer able to maintain this.
In order to sustain the resources needed to complete our testing services, quality, and delivery commitments, we will be raising our method portfolio prices by 6.5% starting in 2023 except for the methods listed below:
- NQA-54.0003 – Pesticide Residue Analysis by Electrospray LC-MS/MS
- NQA-54.0005 – Pesticide Residue Analysis by GC-MS/MS
- LI-00.848 – Trace Elements Analysis by ICP-MS
- LI-00.185 – Mycotoxins in Foodstuffs by LC-MS/MS
- DOP-759-1003-1 – Identification of unknown microorganisms
We greatly appreciate your business and understanding during these challenging times!
Method Implementation at NQAC Dublin
Threats to food safety and regulatory compliance are constantly changing, and the methods for detecting those threats must evolve. New method implementation is a carefully planned and managed project that requires multi-departmental collaboration to succeed.
Multiple sources drive new methods, creating an ever- evolving review of resource management and technical assessment. Major contributors can be internal, such as cost and turnaround time reduction, but external drivers should also be considered. These sources can include customer requests, emerging regulations, anticipated threats to food safety, or market crisis support.
The decision to implement a new method in the laboratory relies on many factors, such as:
- customer demand, expected sample volumes
- cost to run the method, labor, purchase of new equipment/supplies
- the need for validation and/or verification
- resources needed (i.e., project personnel, IT resources)New method implementation is not always a simple process. The least complex implementations take as little as one month. The most complex can extend to a year or more. One of the first steps in the process is to research if there are already methods to choose from (there might be cases where multiple options are available) and evaluate each platform to determine fitness-for-purpose, ease of use, and cost of running the method.
Once the potential new method is chosen, our laboratory will evaluate its performance capabilities. The method must meet the performance criteria established via in-house validation or verification studies. Criteria includes: determining the levels of detection, demonstrating that a sample matrix can be pooled, sample preparation assessments for routine and challenging matrices, limit of quantification, accuracy profiles/method equivalency, estimated bias, as well as other criteria defined in the validation/verification guidelines (i.e., ISO 16140, AOAC). If a method does not meet the performance criteria, then it may not be fit-for-purpose and implementation may not be pursued.
In the case there are no methods available that can meet the needs, Nestlé laboratories will start a new method development to expand the capabilities of the analysis. There are cases in which new techniques or technologies may be required, and scientific original equipment manufacturer (OEM) vendors may be brought into partner on developing of a solution. Sourcing of the instrumentation, equipment, consumables, and reference materials are established to ensure that the method can be performed reliably. If new vendors are needed, evaluations will determine if they meet Nestlé’s standards before they’re onboarded.
The core requirement of a new method implementation is the validation of the method performance.
Demonstration that the results can meet the customer needs and at the same time hold up to scientific scrutiny are non-negotiables in the world of food safety and quality management. Additional work by the QA team is performed to determine if the method meets ISO accreditation requirements for inclusion within the scope of the portfolio.
Once the potential new method has been proven to deliver the needed results within the designed quality parameters, the next step for the laboratory business and support functions is to identify staff, equipment, laboratory space and time resources to satisfy the sample volume and turnaround time.
The last major consideration is the design, development and testing of the new method in the laboratory’s information management system (LIMS). Extensive programming and testing are required to create standards and reagents, the method itself and its associated result components, calculations, result formatting, report generation, invoicing, as well as communication with other software services (i.e., SAP). The more complex a new method is, the greater amount of time is required to ensure proper development and functionality of the LIMS system. Additional consideration is made for the staff training, safety/environmental considerations, sample storage, waste generation, and other impacts for the roll out of a successful method.
Once a new method is successfully implemented and live in the lab, a period of “hypercare” is performed to ensure all aspects of the method are functioning correctly.
This may involve ensuring the method is performing as intended, equipment and consumables are functioning properly, LIMS systems are calculating results correctly and successfully sending out final reports, etc. The hypercare period usually lasts for about one to two months, and any issues encountered are swiftly resolved.
Even after the hypercare period ends, the method will be continually monitored as it is used through process controls or spikes, troubleshooting as issues occur, proficiency testing performance, as well as internal or external audits to ensure conformance to ISO 17025. In some cases, additional food matrices may need to be validated and verified by the laboratory (with vendor support, if needed) if those matrices fall out of scope of the method. The need for additional matrix validation may arise by customer demand and/or development of new food products.
New method implementation projects can be challenging processes for the laboratory, dependent on the complexity of the method, resource availability, and performance of the method. In our experience, each method implementation project is unique and has its specific set of challenges. But with these challenges, it provides the laboratory valuable experience on how to overcome these challenges. Successful method implementation requires effective collaboration between laboratory departments, and support from vendors and technical experts.
NQAC Dublin is here support your business. Click here to access our current Analysis Portfolio. If you have any questions or need assistance with your analytical needs, please email our Customer Service team at nqacdublincustomerservice@us.nestle.com.
World Food Safety Day






Vicine and Convicine Testing in Fava Bean Derived Ingredients
Over the past few years, the popularity of plant-based foods has skyrocketed leading to an explosion of new product development, innovation, and brand launches in this category. Recent data commissioned by The Good Food Institute shows that retail sales of plant-based foods intended to replace animal-based foods have grown over 30% in a two-year period, reaching nearly $4.5 billion in sales as of 2019.1
With this growing interest in vegan and vegetarian foods, manufacturers are investigating plant-based proteins to use in product development.
Fava beans (Vicia faba) have been increasingly used in the development of these alternative protein sources and have been suggested as an alternative to soybeans based on its potentially smaller environmental impact.2 Fava beans are adapted to a wide range of global agro-ecological zones which reduces greenhouse gas emissions that result from transport over long distances. They are also able to symbiotically fix nitrogen which reduces the reliance on nitrogen inputs into the soil and allows for improved soil fertility.3 From the product development aspect, fava beans provide color, taste and texture that is competitive with soy and other plant-based protein alternatives. This makes it an attractive option due to its bright color and neutral taste.2
Despite the positive aspects of using fava beans as an alternative protein, the potential presence of vicine and convicine has traditionally restricted its use.
Vicine and convicine are alkaloid β-glycosides found in fava beans (Vicia faba) that are hydrolyzed to their aglycones (divicine and isouramil) in the colon. These two aglycones are toxic to individuals who suffer from hereditary loss of the enzyme glucose-6-phosphate dehydrogenase. This toxicity develops into a potentially fatal form of anemia known as favism.
This risk can be mitigated through adequate processing and treatment, but it is also essential that raw material and finished products are monitored for the presence of these compounds. NQAC Dublin recently implemented a method that allows for the quantitative determination of vicine and convicine in fava beans and food products containing fava bean derived ingredients. We utilize UPLC-UV technology with a BEH-HILIC column to analyze vicine and convicine with a quantifiable limit of 20 mg/kg for raw materials. Finished products can be tested with a quantifiable limit of 2 mg/kg, however at this time these products are not fully validated with the method.
If you have questions or would like to discuss testing and your needs, reach out to nqacdublincustomerservice@us.nestle.com. We’d be happy to answer any questions you have.
- https://www.forbes.com/sites/douglasyu/2020/01/19/plant-based-foods-are-hot-now-they-just-got-hotter/?sh=11816f13214c
- https://www.foodingredientsfirst.com/news/are-fava-beans-the-new-soy-danish-scientists-highlight-crop-that-bursts-with-protein-296799.html
- http://centaur.reading.ac.uk/88530/8/1-s2.0-S0924224419301992-main.pdf
5 Questions about Rapid Mold & Yeast Testing
NQAC Dublin has a new Mold & Yeast method that will report results out in 3 days, rather than the 5 days required for the traditional methods. Below are five of our most frequently asked questions.
Why is it important to test for Mold and Yeast at my facility?
While not all molds or yeasts on food are dangerous, mold is an obvious and unappealing visual indicator of poor quality to a consumer. Both mold and yeasts can contribute to negative physical, chemical, and sensory properties of the food product.
Molds produce spores, which easily spread through the air, and its presence in a facility can quickly become a large-scale contamination issue.
Some molds (Aspergillus, Byssochamlys, Penicillium) can produce mycotoxins, and high levels of mold in a product could indicate this potentially deadly risk.
In yeast fermented products, enumeration of recoverable yeast can indicate what stage in the fermentation process the product is in.
What are the advantages of the Rapid Mold & Yeast method by Symphony agar over traditional methods?
Traditional mold/yeast methods only rely on the low pH of the agar to select against some bacteria. There are many groups of bacteria, however, that can grow at a low pH (lactic acid bacteria, in particular) and could out compete the target molds or yeasts, or even be mistaken for yeast. Symphony agar contains antibiotics to further reduce potential bacterial growth. This can be very beneficial for products with a high microbial load to provide a more accurate mold and yeast count.
Symphony agar also contains growth promoters to allow mold and yeast results to be counted and reported at 3 days instead of 5 days. This shorter time period gets results reported more quickly and reduces the risk of unreadable plates from common molds that spread over time.
Studies at NQAC for method LI-00.753 implementation showed comparable recovery of target organisms to NQA-00.4100, while providing the above benefits.
The studies showed comparable recovery of osmophilic mold and yeast strains to NQA-00.4420 in high sugar products. However, equivalency to this method has not yet been fully evaluated.
Can this method tell me what type of mold or yeast is present?
LI-00.753 cannot determine specific mold or yeast organisms, however the shorter incubation period does allow for faster notification of mold and yeast presence; identification testing can be requested and initiated sooner. If mold or yeast identification will be required, please make sure to include this information with the sample submission to ensure the plates are retained. Some yeasts may be identified in-house using our MALDI-TOF platform, otherwise samples can be sent externally for further typing.
What types of things can I test?
Symphony agar allows the enumeration of yeasts and molds in all human and animal food products regardless of their water activity. It can also be used for environmental samples in production areas. Studies for method LI-00.753 implementation utilized various routine and challenging sample types, including dairy, confections, cocoa, coffee, high salt items, high sugar items, garlic, spices, flavorings, and more.
How will my results be reported?
LI-00.753 can be reported as mold only, yeast only, or both mold and yeast. If both mold and yeast are requested, you will receive a mold result and a yeast result, not a total number of organisms.
Updates to our Method Portfolio
Lower Limit of Quantitation: Arsenic Speciation by ICP-MS
Our Arsenic Speciation method will now be offered with revised Limit of Quantification (LOQ) of 5 µg/kg in liquid products and 10 µg/kg in dry products (defined as the sum of the As (III) and As (V) species).
The proposed limits for inorganic arsenic in the 2021 Baby Food Safety Act of 2021, if enacted, would lower the current allowable levels in baby food and will require laboratories to meet this new need.
To meet this new challenge, we have lowered limits and will continue to work to meet the future needs for our infant and toddler food customers.
The Baby Food Safety Act of 2021
Ethylene Oxide (Total) – EO
Our new internal offering of Ethylene Oxide (Total) by GC-MS/MS will support submissions of high and low water content samples. Some examples are ice cream, herbs, vegetables, sauces, cereals, and thickeners.
The limit quantification equals 0.010 mg/kg for most matrices; however may increase of 0.025/0.050 mg/kg depending on matrix complexity.
Recent Publication
Towards a consensus LC-MS/MS method for the determination of acrylamide in food that prevents overestimation due to interferences
NQAC Dublin is honored to have Chemist Ashley Griffin as a co-author in the recent publication in the journal Food Additives & Contaminants (1)
Griffin, a member of the AOAC working group on acrylamide analysis, led work performed at NQAC Dublin as a part of a collaborative multi-lab study to determine causes of over estimation in acrylamide measurement. Previously, N-acetyl-ß-alanine was identified as an interference that could create mis-quantitation in a wide variety of food matrices. This work follows up with an evaluation of methods that eliminate the bias that is created by this compound.
This work is important to laboratories, food producers, and consumers alike, as reliable measurement is fundamental to building confidence in any quality assurance approach and allows food producers to closely control their process to minimize risk and ultimately protect the consumer.
The U.S. National Toxicology Program considers acrylamide to be a human health concern as it has been shown to cause cancer in animals in high dose exposures. The European Union has also set benchmarks for acrylamide levels in certain food commodity groups and maximum residue levels in certain foods are currently being considered.
Acrylamide formation occurs during the cooking process of starchy foods when asparagine and reducing sugars are heated above 120°C through the Maillard reaction (2). This process contaminant makes products made from potatoes, cereal grains, and coffee susceptible to acrylamide formation during cooking. While some acrylamide formation is inevitable, producers can employ a variety of approaches to limit the formation through raw material selection and control of cooking processes (3).
If you have any questions about acrylamide testing in your products or raw materials, please reach out to NQAC Dublin Customer Service and we would be glad to help!
References:
(1) Aurélien Desmarchelier, Aude Bebius, Frédérique Reding, Ashley Griffin, Marta Ahijado Fernandez, Jason Beasley, Emilie Clauzier & Thierry Delatour (2022) Towards a consensus LC-MS/MS method for the determination of acrylamide in food that prevents overestimation due to interferences, Food Additives & Contaminants: Part A, Full article: Towards a consensus LC-MS/MS method for the determination of acrylamide in food that prevents overestimation due to interferences (tandfonline.com)
(2) Raffan S, Halford NG. 2019. Acrylamide in food: progress in and prospects for genetic and agronomic solutions. Ann Appl Biol. 175(3):259–281.
(3) U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition “Guidance for Industry – Acrylamide in Foods”, March 2016
Upcoming Publication
Chlorate and perchlorate – LC-MS/MS analytical method validation in a
broad range of food commodities
NQAC Dublin collaborated with three other NQAC locations and Nestlé Research in evaluating an analytical procedure for the routine monitoring of chlorate and perchlorate in a wide range of food matrices. This study will be published in an upcoming issue of Microchemical Journal and is based on the perchlorate/chlorate method currently ran at NQAC Dublin.
Please join us in congratulating one of our chemists, Ashley Griffin, in this awesome achievement!
Analysis Portfolio Updates
New Method
Alternaria Toxins
Our new internal offering of Alternaria Toxins monitors five mycotoxins produced by the Alternaria fungi and is applicable to a wide variety of foods.
The method has been validated on cereals, tomato-based products, tree nuts, vegetable oils, dried fruits, spices, cocoa, green coffee, herbs, and tea.
Samples are are prepared using a QuEChERS type extraction and the analysis employs liquid chromatography coupled with tandem mass-spectrometry detection. Quantitation limits vary by matrix type and analyte, please refer to the below table for more information.
New Test Packages
Nutritional Composition Package
This test package includes the 8 major macronutrients needed for nutritional composition testing.
Extended Nutritional Composition Package
The Extended Nutritional Composition package includes the 8 major macronutrients as well as Fiber, Salt, and Sodium.
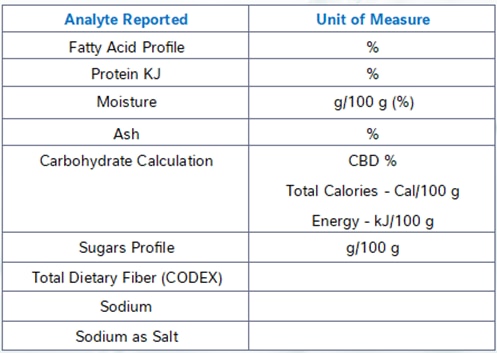
Veterinary Drugs Panel
This test package combines the four veterinary drugs methods we offer into one easy to select package. This option will allow you to ensure your veterinary drug testing needs are fully covered.
Nutritional Tests Package
The Nutritional Tests Package contains all of the analyses in the Nutritional Labeling package but without the label creation.
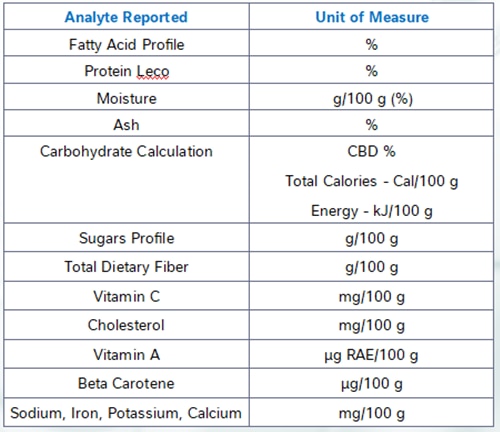
Dioxins and related compounds: What are they?
By: Darren Lease, Chemist and Andrew Savage, Expert Chemist
Dioxin and related compounds, known as PCDDs, PCDFs, and PCBs, are persistent organic pollutants (POPs) that are of concern due to their toxicity and chemical stability. These compounds are chemically related substances (congeners) that differ in the number and location of chlorine atoms on aromatic rings.
Dioxins are by-products of natural processes such as forest fires, and industrial processes including smelting, waste incineration, and the manufacturing of some herbicides and pesticides. Current technologies and approaches have reduced dioxin emissions significantly, but because of the persistence of these compounds in the environment, dioxins remain a threat to the food supply long after they are created1.
These compounds are also persistent once they enter the body as they tend to accumulate in fat tissues. Because of this, dioxins accumulate at higher levels moving up the food chain. Elevated exposures in the short term can cause chloracne, a rare skin eruption of blackheads, cysts and nodules2, and altered liver function. Long term exposure is linked to issues with the immune & nervous systems and TCDD (2,3,7,8 -Tetrachlorodibenzo-p-dioxin) is classified by IARC as a “known human carcinogen”.
The measurement and reporting of Dioxins are evaluated with a toxicity equivalency quotient (TEQ) that evaluates the toxicity of the mixture of compounds. To facilitate this, each congener is assigned a toxicity equivalency factor (TEF) which is the ratio of estimated toxicity for a particular congener to the toxicity of the most toxic compound: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD). This testing is performed by Gas Chromatography-Mass Spectrometry after an extensive and specialized sample preparation.
In 2022, NQAC Dublin will be able to offer dioxins analysis on a variety of sample matrices.
Reach out to us at nqacdublincustomerservice@us.nestle.com for assistance in setting up future testing or for any additional questions.
References:
- https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health
- https://www.publichealth.va.gov/exposures/agentorange/conditions/chloracne.asp#:~:text=Chloracne%20is%20a%20rare%20skin,from%20more%20common%20skin%20disorders.
Chlorpyrifos tolerances set to expire for all commodities
By: Ke Du, Chemist and Andrew Savage, Laboratory Supervisor – Contaminants
Chlorpyrifos is an organophosphate insecticide commonly used on a wide variety of crops including corn, soybeans, grapes, apples, grains, and vegetables. It has been widely used in the U.S., with the USDA estimating that 3 to 5 million kg have been applied since it was introduced in 1965. Chlorpyrifos works by interrupting insect nervous systems and is effective against a wide range of invertebrate pests.
Usage for the chemical has declined in recent years as California placed significant restrictions on its use and the EU banned its sale in 2020. Additionally, increased pressure to address the neurotoxicity of the chemical has been raised to the U.S. Environmental Protection Agency by various groups over the last decade due to the potential effects on children and farm workers1.
On April 29th 2021, a federal appeals court ruled that the US must ban all uses of chlorpyrifos on food, or establish new residue levels for the insecticide that are safe for children. This final court rule became effective October 29, 2021 and as a result, the tolerances for all commodities expire on February 28, 20222.
Additional information regarding this final rule can be found at:
Frequent Questions about the Chlorpyrifos 2021 Final Rule | US EPA
NQAC Dublin can assist in screening for Chlorpyrifos using state-of-art of liquid chromatography/tandem mass spectrometry instruments. Our NQA-54-0003 method offers testing to a limit of quantitation of 10 µg/kg for a wide range of food matrices (50 µg/kg for spices & difficult matrices) to help fulfill your regulatory & food safety requirements.
Please reach out to our Customer Service team at nqacdublincustomerservice@us.nestle.com for assistance in setting up testing or for any additional questions.
References:
Identification of newly recognized Listeria species at NQAC Dublin
By: Jeff Baldwin, Microbiologist
The genus Listeria currently includes 26 recognized species and 6 subspecies [1]. Recent technological advances in whole genome sequencing have led to the discovery of many of these species. In addition, this technology has allowed researchers to divide the species into two clades, or groups in which the members are genetically similar and likely evolved from a common ancestor [2]. The first clade – Listeria sensu stricto – is the clade of interest to public health since it includes the well-known food pathogen L. monocytogenes that causes listeriosis in humans [3]. Therefore, detection of non-monocytogenes sensu stricto species is considered an indicator for an increased risk of L. monocytogenes contamination [3]. The other clade – Listeria sensu lato – is found in the environment but is unable to colonize mammalian hosts due to missing genes involved in gastrointestinal infection [3].
Since 2009, the members of the Listeria sensu lato group (except for L. grayi) have been newly described through whole genome sequencing. They are unidentifiable by traditional biochemical methods. However, advanced tools have been developed that improve the accuracy in identifying organisms in the expanding Listeria genus [4]. One such tool is the Bruker MALDI Biotyper System. It uses MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time of Flight) mass spectrometry to determine the unique fingerprint of an organism based on a characteristic pattern of highly abundant proteins [5]. This pattern is then used to identify the organism by matching it with an extensive and continuously updated reference library [5].
Here at NQAC Dublin, the MALDI Biotyper has allowed us to accurately identify two Listeria species that are not commonly found in food.
The first of which, L. marthii, was first identified in 2010 and is in the Listeria sensu stricto clade. L. marthii is sometimes misidentified as L. innocua using traditional biochemical tests [2]. The other unusual species we identified, L. newyorkensis, was first identified in 2015 and is in the Listeria sensu lato clade, so it is generally not considered a threat to public health [2]. L. newyorkensis would be likely misidentified as L. grayi using traditional biochemical tests since L. grayi is the only member of Listeria sensu lato that is identifiable by these methods [2].
These recent advancements have allowed NQAC Dublin to provide our customers with accurate Listeria test results so that informed decisions about food quality and safety can be made.
Please reach out to us at nqacdublincustomerservice@us.nestle.com if you have any questions and we would be happy to help.
References
[5] https://www.bruker.com/en/products-and-solutions/microbiology-and-diagnostics/microbial-identification/_jcr_content/root/contentpar/linklist.download-asset.pdf/primarylist/item0/1884563-brochure-maldi-biotyper-ruo-gp-bruker-md-12-2020.pdf
Veterinary Drugs: What are they and why should you test for them?
Veterinary drugs are classified by The Codex Alimentarius Commission as “any substance that is applied or administered to any food-producing animal, whether used for therapeutic, prophylactic, or diagnostic purposes, or for the modification of physiological functions or behaviors. These drugs are not limited to just antibiotics and wormers (anthelminthics); sedatives, painkillers, anti-inflammatories, dyes and substances with anabolic effects are also considered veterinary drugs.1 These drugs are often subject to regulations globally, that can vary based on the regulatory body that the product is subject to. These food regulations determine if there is threshold for a particular veterinary drug compound in products intended for consumption.
Consumers and food manufacturers often consider the impact that these substances may have on meat or milk-based products, but they do not always consider that veterinary drugs are also used in the fish and honey industries. For this reason, it is important to include products that include fish or honey ingredients in your testing plan. Depending on the regulation, the detection of these analytes may not pose a regulatory or health concern. However, they can have an impact on consumer perception. This risk can be mitigated through a strong testing approach, ensuring that the analytical methods used are appropriate and valid, and that your materials are well characterized.
To learn more about the importance of validation parameters, test design, and the different types of testing available, register to view on demand.
References:
- https://www.govinfo.gov/content/pkg/FR-2020-03-09/html/2020-04749.htm
September is Food Safety Education Month
This month, we are celebrating Food Safety Education with a series of videos from our experts. We look forward to sharing an FAQ and fun fact to spotlight some of our laboratories at NQAC Dublin.
Check out our Vitamins Laboratory Spotlight Video here. And remember to visit this page over the next few weeks for additional videos from our team.
*UPDATE: Now you can also check out our Contaminants Laboratory Spotlight Video here.
If you have any questions or need our testing support for your business, don’t hesitate to ask: nqacdublininfo@us.nestle.com
Tips for a Successful Environmental Monitoring Program
By: Andrea Chmelar, Microbiology Laboratory Supervisor
An Environmental Monitoring Program (EMP) is critical for understanding the hygienic state of your facility. When done correctly, it provides valuable insight on how well your Good Manufacturing Practices and pre-requisite programs operate. But, just going through the motions is time-consuming and costly. Critical thinking and committed data analysis help to drive strategic improvements. Below are some tips for operating a successful EMP:
Not all buffers are equal.
The right buffer is compatible with your sanitizer, testing platform and has the correct timeline for viability.
Use the appropriate sampling device.
The type and material you choose make a difference. Swabs and sponges both offer different advantages and disadvantages. Educate yourself on which is most appropriate for your facility.
For dependable and accurate results, consistency is imperative.
Be consistent with sample surface size, especially for hygiene indicators like aerobic plate count, Enterobacteriaceae, and E. coli.
Pay attention to the potential for biofilms.
Pressure during sampling and scrubbing is key to breaking these up. Several swabs are now available that are more durable and will stand up to heavy scrubbing.
Seek and destroy!
Often employees are afraid to find pathogens and choose areas that are easy to clean or less prone to contamination. Remember that if a pathogen is present, you want to know. Taking immediate action to eliminate, control, or reduce the issue will keep a small problem from turning into a big one.
Evolve.
You and your team work hard to collect all the information from your EMP. Track results in an Excel or system and use heat maps for a visual of the hygiene in your facility. Revisit your program at least yearly.
Finally, time and temperature are often critical control points in the manufacturing process. They also ensure quality for sticks and swabs. Do not let all your hard work go to waste because you sent out your samples mistakenly. Both can impact the viability of your swabs as significant die-offs can occur. Swabs and sponges should be shipped under the proper conditions to maintain a temperature of 0-10°C. Check with your manufacturer for the timeline most appropriate for your sampling tool.
If you need assistance with your program, or have questions, reach out to us at We will be happy to answer any questions you may have.
No Price Increase for 2022
NQAC Dublin is here to help you prepare for next year’s testing budget. We are proud to announce there will not be an overall percent price increase applied to our methods in 2022. For questions, prices can be found using our portal or contact us at nqacdublincustomerservice@us.nestle.com.
Analysis Portfolio Updates
Method Updates
Trace Elements Quantification Limit Changes
Our Trace Elements (Heavy Metals) method now offers lower QLs on Aluminum, Arsenic, Cadmium, Lead, and Mercury components. With this change, we now match the QL requirements in the Baby Food Safety Act of 2021.
Below you will find a quick view of the current and future QLs:
Updates to Total Oligosaccharides Method
Our Total Oligosaccharides method provides new test variations that offer flexibility based on product type and the oligosaccharide present. There are 4 testing options now available, as well as two new calculations:
- GOS Vivinal Profile
- GOS Clasado Profile
- GOS BMOS Profile
- GOS BMOS excluding 2FL, 6SL, LNT, LNnT
- GOS Disaccharides (calculation)
- Total GOS excluding Disaccharides (calculation)
New Method
Ergot Alkaloids
Our Ergot Alkaloids method provides the quantitative determination of 12 ergot alkaloids and their sum (Ergot Alkaloids Total) by lLC-MS/MS.
The method has been validated on raw cereals (wheat, corn, rice, barley, rye, oat, triticale, millet, sorghum, and spelt), infant cereals, whey protein powder and cereal-based baby foods with a limit of quantification on each reported analyte of 0.5 µg/kg (sum 6.0 µg/kg).
Shelf-Stable Foods and Bacterial Spoilage
Shelf-stable foods are non-perishable products that can be safely stored at room temperature1. These products typically have undergone some preservation process and are considered “commercially sterile”. While commercially sterile products will not spoil or cause illness under normal circumstances, the products are not completely sterile. They may be subject to some form of bacterial spoilage. For canned products, spoilage may be caused by a range of factors including insufficient thermal processing and post-process contamination.
Insufficient thermal processing generally causes spoilage through bacterial spore growth, either with or without gas production. When the outgrowth of spore-forming bacteria results in acid production without gas production this is known as “flat-sour” spoilage2. When gas production is present, this can cause bloating and rupturing of the finished product packaging. Spoilage due to thermal processing insufficiency is characterized by the presence of a pure culture of spore forming microorganisms.
Post-process contamination is characterized by the presence of a mixed culture of spore-formers and different vegetative flora. This spoilage can be caused by defective seams, dirty conveyor systems, or poor operation of filled can handling equipment3. In both cases, a physical examination of container integrity should be completed in addition to examination for viable organisms. This provides a better understanding of where in the process the issue may have occurred.
For both insufficient thermal processing and post-process contamination, further investigation is needed to identify the root cause of the issue. This should include examination of full lot information and data for trends and patterns before arriving at any conclusions.
If you need assistance with laboratory evaluation of spoilage, NQAC Dublin is here to help. We offer commercial sterility testing to help ensure your thermal processing is effective. Also, spoilage evaluations are provided if potential spoilage in a product is noted. Please reach out to us at nqacdublincustomerservice@us.nestle.com and we would be happy to provide answer any questions you may have.
References
https://ask.usda.gov/s/article/What-does-shelf-stable-mean
A. Zottola, SPOILAGE | Bacterial Spoilage, Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003, Pages 5506 through 5510. (https://www.sciencedirect.com/science/article/pii/B012227055X011287)
Henriëtte M. C. Put, H. J. Witvoet, and W. R. Warner, Mechanism of Microbiological Leaker Spoilage of Canned Foods: Biophysical Aspects, Journal of Food Protection, Vol. 43, No. 6, Pages 488-497. (https://meridian.allenpress.com/jfp/article/43/6/488/205581/Mechanism-of-Microbiological-Leaker-Spoilage-of)